Silenes
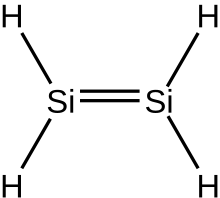
Silene, or silalkenes,[1] are unsaturated hydrosilicons, which means that they consist only of hydrogen and silicon atoms and all bond, with the exception of one[citation needed] double bond, are either single or double bonds. By definition cycles are excluded, so that the silenes comprise homologous series of inorganic compounds with the general formula Si
nH
2n - 2k + 2, k > 0, where k is defined as the number of double bonds. There are no commercial sources.
Each silicon atom has three or four bonds (either Si-H, Si-Si, or Si=Si), and each hydrogen atom is joined to a silicon atom (H-Si bonds). A series of linked silicon atoms is known as the silicon skeleton or silicon backbone. The number of silicon atoms is used to define the size of the silene (e.g. Si2-silene).
A silenyl group, is a functional group or side-chain with the general formula Si
nH
2n - 2k + 1, k > 0, where k is defined as the number of double bonds, for example an disilenyl or trisil-1-en-1-yl group.
The simplest silene is (the parent molecule) is disilene, Si
2H
4. There is no limit to the number of silicon atoms that can be linked together, the only limitation being that the molecule is acyclic, is unsaturated, and is a hydrosilicon.
For bridged silenes, the Bredt's rule states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough (8 or more atoms). Aromatic compounds are often drawn as cyclic silenes, but their structure and properties are different and they are not considered to be silenes. Although silenes are mainly of theoretical interest, substituted silenes are well known class of organosilicon compounds.
Structure[]
Like single covalent bonds, double bonds can be described in terms of overlapping atomic orbitals, except that, unlike a single bond (which consists of a single sigma bond), a silicon-silicon double bond consists of one sigma bond and one pi bond. This double bond is stronger than a single covalent bond and also shorter.
Each silicon of the double bond uses its three sp2 hybrid orbitals to form sigma bonds to three atoms. The unhybridized 3p atomic orbitals, which lie perpendicular to the plane created by the axes of the three sp² hybrid orbitals, combine to form the pi bond. This bond lies outside the main Si—Si axis, with half of the bond on one side and half on the other.
Rotation about the silicon-silicon double bond is not restricted because it involves breaking the pi bond, which requires a small amount of energy. As a consequence, substituted alkenes may not exist as one of two isomers.
It is certainly possible to twist a double bond.
History[]
The first transient disilene was synthesized in 1972 by D. N. Roark and Garry J. D. Peddle. The first thermally stable disilene, tetramesityldisilene, was described in 1981 by West, Fink and Michl.[2][3] It was prepared by UV-photolysis of the related cyclic trisilane:
- 2 [Si(mesityl)2]3 → 3 (mesityl)2Si=Si(mesityl)2
Properties[]
Simple disilenes are very reactive species and easily undergo polymerization or other reactions, so their life is very short. To prevent this polymerization and other reactions, bulky substituents are used to stabilize disilenes effectively for long-term survival in dilute solution and even in crystals.
Stable disilenes are generally yellow- or orange-colored crystalline compounds.
The Si=Si double bond lengths of disilenes vary between 2.14 and 2.29 Å and are nearly 5 to 10% shorter than the Si-Si single bond lengths of corresponding disilanes. This rate of bond shortening is less than ca 13% in carbon compounds, but is short enough for true double bond characteristics.
A further peculiarity of disilenes is the trans-bending of the substituents, which is never observed in alkenes. The trans-bent angles of disilenes between the R2Si planes and the Si=Si vector range from 0 to 33.8 °. This distortion is rationalized by the stability of the corresponding silylene fragments, although disilenes do not typically dissociate.
The distorted geometry of disilenes can be rationalized by considering the valence orbitals of silicon, which are 3s and 3p, whereas those of carbon are 2s and 2p. Thus, the energy gap between the ns and np orbitals of a silicon atom is larger than that of a carbon atom. Therefore, silylene fragments are in a singlet state, while carbene fragments are in a triplet state. So, when double bonds are formed by the interaction of these two fragments, disilenes which consist of two silylene units are trans-bending and alkenes which consist of two carbene units are planar. The bending is even more extreme for the tin analogues of disilenes.[1]
Synthesis[]
Disilenes are generally synthesized by reduction of 1,2-dihalodisilane, by retro-Diels–Alder fragmentation, by dimerization of silylenes, by photofragmentation of cyclopolysilanes, or by rearrangement of silylsilylenes.
A series of 1,1,1,4,4,4-hexaalkyl-2,3-bis(trialkylsilyl)tetrasil-2-enes structural analogues are typically synthesised using reductive coupling of the corresponding 1,1,1,3,3,3-hexaalkyl-2,3-tribromotrisilanes.
Nomenclature[]
IUPAC names[]
To form the root of the IUPAC names for silenes, simply change the -an- infix of the parent to -en-. For example, SiH
3-SiH
3 is the silane disilANe. The name of SiH
2=SiH
2 is therefore disilENe.
In higher silenes, where isomers exist that differ in location of the double bond, the following numbering system is used:
- Number the longest silicon chain that contains the double bond in the direction that gives the silicon atoms of the double bond the lowest possible numbers.
- Indicate the location of the double bond by the location of its first silicon.
- Name branched or substituted silenes in a manner similar to silanes.
- Number the silicon atoms, locate and name substituent groups, locate the double bond, and name the main chain.
Organosilenes[]
The organosilenes are a group of chemical compounds derived from silenes containing one or more organic groups. They are a subset of the general class of organosilicons, although the distinction is not often made.
See also[]
References[]
- ^ Jump up to: a b Philip P. Power "pi-Bonding and the Lone Pair Effect in Multiple Bonds between Heavier Main Group Elements" Chemical Reviews, 1999, 99, 3462. doi:10.1021/cr9408989
- ^ West, R.; Fink, M. J.; Michl, J. Tetramesityldisilene, a Stable Compound Containing a Silicon-Silicon Double Bond Science 1981, Vol. 214, pp. 1343-1344. doi:10.1126/science.214.4527.1343.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 363. ISBN 978-0-08-037941-8.
- Silicon compounds