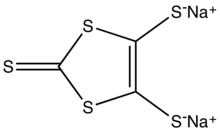
Sodium 1,3-dithiole-2-thione-4,5-dithiolate
| |
| Names | |
|---|---|
| Preferred IUPAC name
Disodium 2-sulfanylidene-2H-1,3-dithiole-4,5-bis(thiolate) | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C3Na2S5 | |
| Molar mass | 242.31 g·mol−1 |
| Appearance | yellow solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium 1,3-dithiole-2-thione-4,5-dithiolate is the organosulfur compound with the formula Na2C3S5, abbreviated Na2dmit. It is the sodium salt of the conjugate base of the 1,3-dithiole-2-thione-4,5-dithiol. The salt is a precursor to dithiolene complexes and tetrathiafulvalenes.[1]
Reduction of carbon disulfide with sodium affords sodium 1,3-dithiole-2-thione-4,5-dithiolate together with sodium trithiocarbonate:
- 4 Na + 4 CS2 → Na2C3S5 + Na2CS3
Before the characterization of dmit2-, reduction of CS2 was thought to give tetrathiooxalate (Na2C2S4).[2]
The dianion C3S52- is purified as the tetraethylammonium salt of the zincate complex [Zn(C3S5)2]2-. This salt converts to the bis(thioester) upon treatment with benzoyl chloride:[3][1]
- [N(C2H5)4]2[Zn(C3S5)2] + 4 C6H5COCl → 2 C3S3(SC(O)C6H5)2 + [N(C2H5)4]2[ZnCl4]
Cleavage of the thioester with sodium methoxide gives sodium 1,3-dithiole-2-thione-4,5-dithiolate:
- C3S3(SC(O)C6H5)2 + 2 NaOCH3 → Na2C3S5 + 2 C6H5CO2Me
Na2dmit undergoes S-alkylation.[5] Heating solutions of Na2dmit gives the isomeric 1,2-dithioledithiolate.
References[]
- ^ a b "4,5-Dibenzoyl-1,3-dithiole-1-thione". Org. Synth. 73: 270. 1996. doi:10.15227/orgsyn.073.0270.
- ^ Dietzsch, W.; Strauch, P.; Hoyer, E. (1992). "Thio-oxalates: Their Ligand Properties and Coordination Chemistry". Coord. Chem. Rev. 121: 43–130. doi:10.1016/0010-8545(92)80065-Y.CS1 maint: uses authors parameter (link)
- ^ G. S. Girolami, T. B. Rauchfuss and R. J. Angelici (1999) Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA.ISBN 0935702482
- ^ W.T.A.Harrison, R.A.Howie, J.L.Wardell, S.M.S.V.Wardell, N.M.Comerlato, L.A.S.Costa, A.C.Silvino, A.I.de Oliveira, R.M.Silva (2000). "Crystal structures of three [bis(1,3-dithiole-2-thione-4,5-dithiolato)zincate]2− salts: [Q]2[Zn(dmit)2] (Q = 1,4-Me2-pyridinium or NEt4) and [PPh4]2[Zn(dmit)2]·DMSO. Comparison of the dianion packing arrangements in [Q]2[Zn(dmit)2]". Polyhedron. 19 (7): 821–827. doi:10.1016/S0277-5387(00)00322-3.CS1 maint: uses authors parameter (link)
- ^ Niels Svenstrup, Jan Becher (1995). "The Organic Chemistry of 1,3-Dithiole-2-thione-4,5-dithiolate (DMIT)". Synthesis. 1995 (3): 215–235. doi:10.1055/s-1995-3910.CS1 maint: uses authors parameter (link)
- Thiolates
- Alkene derivatives
- Sodium compounds