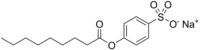
Sodium nonanoyloxybenzenesulfonate
| |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium 4-(nonanoyloxy)benzene-1-sulfonate | |
| Other names
4-Sulfophenyl nonanoate sodium salt; Sodium p-nonanoyloxybenzenesulfonate; p-(Nonanoyloxy)benzenesulfonic acid sodium salt; p-Sodiosulfophenyl nonanoate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| Abbreviations | NOBS |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H21NaO5S | |
| Molar mass | 336.38 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium nonanoyloxybenzenesulfonate (NOBS) is an important component of laundry detergents and bleaches. It is known as a bleach activator for active oxygen sources, allowing formulas containing hydrogen peroxide releasing chemicals (specifically sodium perborate, sodium percarbonate, , sodium persulfate, and urea peroxide) to effect bleaching at lower temperatures.[1]
Synthesis[]
NOBS is formed by the reaction of nonanoic acid (or its esters) with phenol followed by aromatic sulfonation using SO3 to form a sulfonic acid at the para-position.
Bleach activation[]
NOBS was developed by Procter & Gamble in 1983[2] and was first used in American laundry detergents in 1988.[3] NOBS is the main bleach activator used in the U.S.A. and Japan.[4] Compared to TAED, which is the predominant bleach activator used in Europe, NOBS is efficient at much lower temperatures. At 20 °C NOBS is 100 times more soluble than TAED in water.[5] When attacked by the perhydroxyl anion (from hydrogen peroxide), NOBS forms the peroxy acid peroxynonanoic acid and releases the leaving group sodium 4-hydroxybenzene sulfonate, which is an inert by-product.
References[]
- ^ Kuzel, P.; Lieser, T. (1990). "Bleach systems". Tenside, Surfactants, Detergents. 27 (1): 23–8.
- ^ Chung, S. Y.; Spadini, G. L. (1983). US4412934.
- ^ Arno Cahn (30 January 1994). Proceedings of the 3rd World Conference on Detergents: Global Perspectives. The American Oil Chemists Society. pp. 64–70. ISBN 978-0-935315-52-3.
- ^ Hirschen, M. (2005). Handbook of Detergents Part C: Analysis. Marcel Dekker. pp. 439–470. ISBN 9780824703516.
- ^ Reinhardt, G.; Borchers, G. (2009). Handbook of Detergents, Part E: Applications. CRC Press. ISBN 9781574447576.
- Cleaning product components
- Benzenesulfonates
- Anionic surfactants
- Organic sodium salts
- Nonanoate esters