Sulfanilic acid

| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Aminobenzene-1-sulfonic acid[1] | |
| Other names | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ECHA InfoCard | 100.004.075 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C6H7NO3S | |
| Molar mass | 173.19 |
| Density | 1.485 |
| Melting point | 288 °C (550 °F; 561 K) |
| 12.51 g/L | |
| Acidity (pKa) | 3.23 (H2O)[2] |
| Related compounds | |
Related sulfonic acids
|
Benzenesulfonic acid p-Toluenesulfonic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry.[3]
Synthesis[]
Sulfanilic acid can be produced by sulfonation of aniline:[4]

Applications[]
As the compound readily forms diazo compounds, it is used to make dyes and sulfa drugs.[3] This property is also used for the quantitative analysis of nitrate and nitrite ions by diazonium coupling reaction with N-(1-Naphthyl)ethylenediamine, resulting in an azo dye, and the concentration of nitrate or nitrite ions were deduced from the color intensity of the resulting red solution by colorimetry.[5]
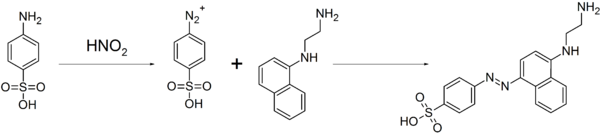
It is also used as a standard in combustion analysis and in the Pauly reaction.
Environmental aspects[]
Reflecting its wide use, sulfanilic acid is found in the leachates of landfills.[6] It is produced by reduction of some azo dyes.[7]
Derivatives[]
- Methyl orange (azo coupling with dimethylaniline)
- Acid orange 7 (azo coupling with 2-naphthol)[8]
- Chrysoine resorcinol (azo coupling with resorcinol)
See also[]
References[]
- ^ Jump up to: a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 789. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The name ‘sulfanilic acid’ is not retained.
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 5–88. ISBN 978-1498754286.
- ^ Jump up to: a b "Sulphanilic acid". A Dictionary of Chemistry. Oxford University Press, 2000. Oxford Reference Online. Oxford University Press.
- ^ Siegfried Hauptmann: Organische Chemie, 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 511, ISBN 3-342-00280-8.
- ^ G. H. Jerffery; J. Bassett; J. Mendham; R. C. Denney (1989). "Colorimetry and Spectrophotometry". Vogel's Textbook of Quantitative Chemical Analysis, 5th Edition. Longman. p. 702. ISBN 0-582-44693-7.
- ^ Holm, John V.; Ruegge, Kirsten.; Bjerg, Poul L.; Christensen, Thomas H. (1995). "Occurrence and Distribution of Pharmaceutical Organic Compounds in the Groundwater Downgradient of a Landfill (Grindsted, Denmark)". Environmental Science & Technology. 29 (5): 1415–1420. doi:10.1021/es00005a039. PMID 22192041.
- ^ Nam, S. (2000). "Reduction of azo dyes with zero-valent iron". Water Research. 34 (6): 1837–1845. doi:10.1016/S0043-1354(99)00331-0.
- ^ Klaus Hunger; Peter Mischke; Wolfgang Rieper; Roderich Raue; Klaus Kunde; Aloys Engel (2005). "Azo Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245..
- Benzenesulfonic acids
- Anilines