Superphenalene
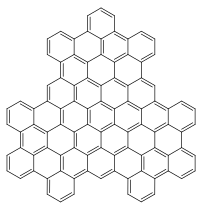 Chemical structure of superphenalene
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dibenzo[u′v′,a′1b′1]benzo[4′′,10′′]anthra[3′′,2′′,1′′,9′′,8′′:1′,12′,11′,10′]tetrapheno[5′,6′,7′,8′,9′:4,5,6,7]tetraceno[2,1,12,11,10,9-uvwxyza1b1]hexaceno[2,1,16,15,14,13,12,11-defghijklmno:3,4,5,6,7,8,9,10-d′e′f′g′h′i′j′k′l′m′n′o′]diheptacene | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| C96H30 | |
| Molar mass | 1183.296 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Superphenalene is a very large polycyclic aromatic hydrocarbon (PAH) with chemical formula C96H30. It can be formally considered to consist of three fused superbenzenes (hexa-peri-hexabenzocoronene).[1]
It can be considered as an overlapping structure of three hexa-peri-hexabenzocoronenes arranged symmetrically around a center.[2][3] These have also been known as building blocks of molecular electronics since 2004 as they form self-assembling columns and nanotubes.[4]
Occurrence[]
It is not known to occur naturally.
Properties[]
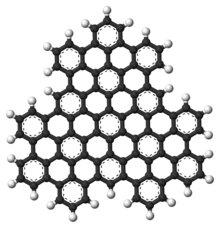
Superphenalene has a planar geometry. With 540,000 mesomeric boundary structures, it has significantly more than hexabenzocoronene (250), (16,100) and also buckminsterfullerene (12,500).[1] The molecule has a threefold symmetry axis perpendicular to the molecule (C3).
References[]
- ^ a b Randić, Milan; Gao, Xiaofeng (1999). "Giant benzenoid hydrocarbons. Superphenalene resonance energy". New Journal of Chemistry. 23 (2): 251–260. doi:10.1039/A808949C.
- ^ Ito, Shunji; Herwig, Peter Tobias; Böhme, Thilo; Rabe, Jürgen P.; Rettig, Wolfgang; Müllen, Klaus (2000). "Bishexa-peri-hexabenzocoronenyl: A 'superbiphenyl'". Journal of the American Chemical Society. 122 (32): 7698–7706. doi:10.1021/ja000850e.
- ^ Wu, Jishan; Watson, Mark D.; Tchebotareva, Natalia; Wang, Zhaohui; Müllen, Klaus (2004). "Oligomers of hexa-peri-hexabenzocoronenes as 'super-oligophenylenes': Synthesis, electronic properties, and self-assembly". The Journal of Organic Chemistry. 69 (24): 8194–8204. doi:10.1021/jo0490301. PMID 15549787.
- ^ Hill, J. P.; Jin, W.; Kosaka, A.; Fukushima, T.; Ichihara, H.; Shimomura, T.; Ito, K.; Hashizume, T.; Ishii, N.; Aida, T. (2004). "Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube". Science. 304 (5676): 1481–1483. Bibcode:2004Sci...304.1481H. doi:10.1126/science.1097789. PMID 15178796. S2CID 39674411.
- Polycyclic aromatic hydrocarbons