Terphenylquinones
Terphenylquinones are fungal dyes from the group of phenyl-substituted p-benzoquinones having the following general structure.[1]
General chemical structure of terphenylquinones
Also derivatives with a central o-benzoquinone structure are known.
Biosynthesis[]
The biosynthesis of terphenylquinones is carried out by dimerization of substituted oxophenylpropanoic acids (phenylpyruvic acids).
Occurrence[]
Terphenylquinones are typical constituents of the boletales.
Examples[]
Bezeichnung Struktur CAS-Nr. Vorkommen Polyporic acid 
548-59-4 Polypore of the order Aphyllophorales, Lichen Sticta coronata[2] Atromentin 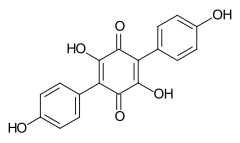
519-67-5 Paxillus atrotomentosus (Basidiomycetes)[3] Aurantiacin 
548-32-3 Hydnellum aurantiacum (Basidiomycetes)[4] Phlebiarubron 
7204-23-1 Cultures of Phlebia strigoso-zonata and Punctularia atropurpurascens (Basidiomycetes)[5] Spiromentin B 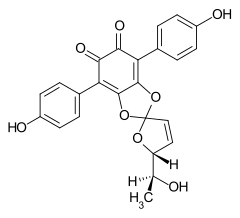
121254-56-6 Paxillus atrotomentosus (Basidiomycetes) and cultures of Paxillus panuoides[6]
See also[]
References[]
- ^ Burkhard Fugmann, ed. (1997), RÖMPP Lexikon Naturstoffe, 1. Auflage, 1997 (in German) (1. ed.), Stuttgart: Georg Thieme Verlag, p. 638, ISBN 3-13-749901-1
- ^ Entry on Polyporsäure. at: Römpp Online. Georg Thieme Verlag, retrieved 3. September 2019.
- ^ Entry on Atromentin. at: Römpp Online. Georg Thieme Verlag, retrieved 3. September 2019.
- ^ Entry on Aurantiacin. at: Römpp Online. Georg Thieme Verlag, retrieved 3. September 2019.
- ^ Entry on Phlebiarubron. at: Römpp Online. Georg Thieme Verlag, retrieved 3. September 2019.
- ^ Entry on Spiromentine. at: Römpp Online. Georg Thieme Verlag, retrieved 3. September 2019.
Categories:
- Quinones
- Aromatic compounds





