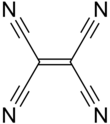
Tetracyanoethylene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ethenetetracarbonitrile | |||
| Other names
TCNE
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.010.527 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6N4 | |||
| Molar mass | 128.094 g·mol−1 | ||
| Density | 1.35 g/cm3 | ||
| Melting point | 199 °C (390 °F; 472 K) | ||
| Boiling point | 130 to 140 °C (266 to 284 °F; 403 to 413 K) 0.1 mmHg (sublimes)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tetracyanoethylene (TCNE) is organic compound with the formula C2(CN)4. It is a colorless solid. It is an important member of the cyanocarbons.
Chittipeddi refers to TCNE as "tetracyanoethenide".[2]
Synthesis and reactions[]
TCNE is prepared by brominating malononitrile in the presence of potassium bromide to give the KBr-complex, and dehalogenating with copper.[1]
Oxidation of TCNE with hydrogen peroxide gives the corresponding epoxide, which has unusual properties.[3]
Redox chemistry[]
TCNE is often used as an electron acceptor. Cyano groups have low energy π* orbitals, and the presence of four such groups, with their π systems (conjugated) to the central C=C double bond, gives rise to an electrophilic alkene. TCNE is reduced by iodide to give the radical anion:[citation needed]
- C2(CN)4 + I− → [C2(CN)4]− + 1⁄2 I2
Magnetic properties[]
Because of its planarity and its ability to accept electrons, TCNE has been used to prepare numerous organic superconductors, usually by serving as a single electron oxidant of an organic electron donor. Such charge-transfer salts are sometimes called Bechgaard salts.[citation needed]
Safety[]
TCNE hydrolyzes in moist air to give hydrogen cyanide and should be handled accordingly.[1]
References[]
- ^ a b c Carboni, R. A. (1963). "Tetracyanoethylene". Organic Syntheses.; Collective Volume, 4, p. 877
- ^ Chittipeddi, Sailesh; Cromack, K. R.; Miller, Joel S.; Epstein, A. J. (1987-06-22). "Ferromagnetism in molecular decamethylferrocenium tetracyanoethenide (DMeFc TCNE)". Physical Review Letters. American Physical Society (APS). 58 (25): 2695–2698. Bibcode:1987PhRvL..58.2695C. doi:10.1103/physrevlett.58.2695. ISSN 0031-9007. PMID 10034821.
- ^ Linn, W. J. (1973). "Tetracyanoethylene Oxide". Organic Syntheses.; Collective Volume, 5, p. 1007
- Alkene derivatives
- Nitriles
- Superconductors