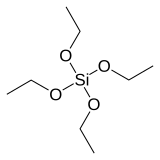
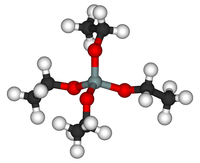
Tetraethyl orthosilicate
| |
| |
| Names | |
|---|---|
| IUPAC name
tetraethoxysilane
| |
| Other names
tetraethyl orthosilicate; ethyl silicate; silicic acid tetraethyl ester; silicon ethoxide; TEOS; tetraethyl silicate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.986 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SiC8H20O4 | |
| Molar mass | 208.33 g⋅mol−1 |
| Appearance | Colourless liquid |
| Odor | Sharp, alcohol-like[1] |
| Density | 0.933 g/mL at 20 °C |
| Melting point | −77 °C (−107 °F; 196 K) |
| Boiling point | 168 to 169 °C (334 to 336 °F; 441 to 442 K) |
| Reacts with water, soluble in ethanol, and 2-propanol | |
| Vapor pressure | 1 mmHg[1] |
| Hazards | |
| Main hazards | Flammable, harmful by inhalation |
| Flash point | 45 °C (113 °F; 318 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
6270 mg/kg (rat, oral)[2] |
LCLo (lowest published)
|
|
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 100 ppm (850 mg/m3)[1] |
REL (Recommended)
|
TWA 10 ppm (85 mg/m3)[1] |
IDLH (Immediate danger)
|
700 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetraethyl orthosilicate, formally named tetraethoxysilane and abbreviated TEOS, is the chemical compound with the formula Si(OC2H5)4. TEOS is a colorless liquid that degrades in water. TEOS is the ethyl ester of orthosilicic acid, Si(OH)4. It is the most prevalent alkoxide of silicon.
TEOS is a tetrahedral molecule. Like its many analogues, it is prepared by alcoholysis of silicon tetrachloride:
- SiCl4 + 4 EtOH → Si(OEt)4 + 4 HCl
where Et is the ethyl group, C2H5, and thus EtOH is ethanol.
Applications[]
TEOS is mainly used as a crosslinking agent in silicone polymers and as a precursor to silicon dioxide in the semiconductor industry.[3] TEOS is also used as the silica source for synthesis of some zeolites.[4] Other applications include coatings for carpets and other objects. TEOS is used in the production of aerogel. These applications exploit the reactivity of the Si-OR bonds.[5] TEOS has historically been used as an additive to alcohol based rocket fuels to decrease the heat flux to the chamber wall of regeneratively cooled engines by over 50%.[6]
Other reactions[]
TEOS easily converts to silicon dioxide upon the addition of water:
- Si(OC2H5)4 + 2 H2O → SiO2 + 4 C2H5OH
An idealized equation is shown, in reality the silica produced is hydrated. This hydrolysis reaction is an example of a sol-gel process. The side product is ethanol. The reaction proceeds via a series of condensation reactions that convert the TEOS molecule into a mineral-like solid via the formation of Si-O-Si linkages. Rates of this conversion are sensitive to the presence of acids and bases, both of which serve as catalysts. The Stöber process allows the formation of monodisperse and mesoporous silica.[7][8][9]
At elevated temperatures (>600 °C), TEOS converts to silicon dioxide:
- Si(OC2H5)4 → SiO2 + 2 (C2H5)2O
The volatile coproduct is diethyl ether.
Safety[]
TEOS has low toxicity by ingestion. While tetramethoxysilane is highly damaging to eyes since it deposits silica, TEOS is much less so due to lower hydrolysis rate of the ethoxy groups.[10]
References[]
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0282". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Ethyl silicate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Bulla, D.A.P; Morimoto, N.I (1998). "Deposition of thick TEOS PECVD silicon oxide layers for integrated optical waveguide applications". Thin Solid Films. 334: 60. Bibcode:1998TSF...334...60B. doi:10.1016/S0040-6090(98)01117-1.
- ^ Kulprathipanja, Santi (2010) Zeolites in Industrial Separation and Catalysis, Wiley-VCH Verlag GmbH & Co. KGaA, ISBN 3527629572.
- ^ Rösch, Lutz; John, Peter and Reitmeier, Rudolf "Silicon Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a24_021.
- ^ Clark, John D. (1972). Ignition! An Informal History of Liquid Rocket Propellants. Rutgers University Press. pp. 105–106. ISBN 9780813507255.
- ^ Boday, Dylan J.; Wertz, Jason T.; Kuczynski, Joseph P. (2015). "Functionalization of Silica Nanoparticles for Corrosion Prevention of Underlying Metal". In Kong, Eric S. W. (ed.). Nanomaterials, Polymers and Devices: Materials Functionalization and Device Fabrication. John Wiley & Sons. pp. 121–140. ISBN 9781118866955.
- ^ Kicklebick, Guido (2015). "Nanoparticles and Composites". In Levy, David; Zayat, Marcos (eds.). The Sol-Gel Handbook: Synthesis, Characterization and Applications. 3. John Wiley & Sons. pp. 227–244. ISBN 9783527334865.
- ^ Berg, John C. (2009). "Colloidal Systems: Phenomenology and Characterization". An Introduction to Interfaces and Colloids: The Bridge to Nanoscience. World Scientific Publishing. pp. 367–368, 452–454. ISBN 9789813100985.
- ^ https://www.mathesongas.com/pdfs/msds/MAT09230.pdf
External links[]
- Ethoxides
- Silicate esters