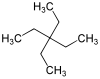
Tetraethylmethane
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3,3-Diethylpentane[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.151.290 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C9H20 | |||
| Molar mass | 128.259 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Odourless | ||
| Density | 724 mg mL−1 | ||
| Melting point | −34 to −30 °C; −29 to −22 °F; 239 to 243 K | ||
| Boiling point | 145.8 to 146.6 °C; 294.3 to 295.8 °F; 418.9 to 419.7 K | ||
Henry's law
constant (kH) |
1.5 nmol Pa−1 kg−1 | ||
| Thermochemistry | |||
Heat capacity (C)
|
278.2 J K−1 mol−1 | ||
Std molar
entropy (S |
333.4 J K−1 mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−6.1261–−6.1229 MJ mol−1 | ||
| Hazards | |||
| NFPA 704 (fire diamond) | 
0
3
0 | ||
| Related compounds | |||
Related alkanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tetraethylmethane is a branched alkane with 9 carbon atoms. It is a highly flammable and volatile liquid at room temperature. It is one of the isomers of nonane.
References[]
- ^ "Tetraethylmethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 11 March 2012.
See[]
External links[]
- Alder, Roger W.; Allen, Paul R.; Hnyk, Drahomír; Rankin, David W. H.; Robertson, Heather E.; Smart, Bruce A.; Gillespie, Ronald J.; Bytheway, Ian (1999). "Molecular Structure of 3,3-Diethylpentane (Tetraethylmethane) in the Gas Phase as Determined by Electron Diffraction and ab Initio Calculations". The Journal of Organic Chemistry. 64 (12): 4226–4232. doi:10.1021/jo981779m.
Categories:
- Alkanes

