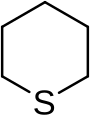
Thiane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Thiane | |||
| Other names
Tetrahydro-2H-thiopyran
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.015.056 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H10S | |||
| Molar mass | 102.1979 | ||
| Appearance | colorless liquid | ||
| Density | 0.9943 g/cm3 | ||
| Melting point | 19 °C | ||
| Boiling point | 141.8 °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Thiane is a heterocyclic compound and an organosulfur compound with the formula (CH2)5S. It is a saturated six-membered ring with five carbon atoms and one sulfur atom. The compound is a colorless liquid. It can be prepared by the reaction of 1,5-dibromopentane with sodium sulfide:[1]
- Br-(CH2)5-Br + Na2S → (CH2)5S + 2NaBr
References[]
- ^ E. V. Whitehead, R. A. Dean, F. A. Fidler "The Preparation and Physical Properties of Sulfur Compounds Related to Petroleum. II. Cyclic Sulfides" J. Am. Chem. Soc., 1951, volume 73, pp 3632–3635. doi: 10.1021/ja01152a022
Categories:
- Sulfur heterocycles
- Six-membered rings
- Heterocyclic compound stubs