Transition-metal allyl complex
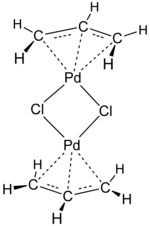
Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.
Examples and their syntheses[]
The allyl ligand is commonly found in organometallic chemistry. Most commonly, allyl ligands bind to metals via all three carbon atoms, the η3-binding mode. The η3-allyl group is classified as an LX-type ligand in the Green LXZ ligand classification scheme, serving as a 3e– donor using neutral electron counting and 4e– donor using ionic electron counting.
An example of a homoleptic allyl complex is Ir(η3-allyl)3.[1] More common are complexes with allyl and other ligands. Examples include (η3-allyl)Mn(CO)4 and CpPd(allyl).
Allyl complexes are often generated by oxidative addition of allylic halides to low-valent metal complexes. This route is used to prepare (allyl)2Ni2Cl2:[2][3]
- 2 Ni(CO)4 + 2 ClCH2CH=CH2 → Ni2(μ-Cl)2(η3-C3H5)2 + 8 CO
A similar oxidative addition involves the reaction of allyl bromide to diiron nonacarbonyl.[4]
Other methods of synthesis involve addition of nucleophiles to η4-diene complexes and hydride abstraction from alkene complexes. Lastly allyl ligands are produced by salt metathesis reactions starting with allyl Grignard and allyl lithium reagents.[1]
Chelating bis(allyl) complexes[]

1,3-Dienes such as butadiene and isoprene dimerize in the coordination spheres of some metals, giving chelating bis(allyl) complexes. Such complexes also arise from ring-opening of divinylcyclobutane. Chelating bis(allyl) complexes are intermediates in the metal-catalyzed dimerization of butadiene to give vinylcyclohexene and cycloocta-1,5-diene.[5]
Sigma-allyl[]
Complexes with η1-allyl ligands (classified as X-type ligands) are also known. One example is CpFe(CO)2(η1-C3H5), in which only the methylene group is attached to the Fe centre (i.e., it has the connectivity [Fe]–CH2–CH=CH2). As is the case for many other η1-allyl complexes, the monohapticity of the allyl ligand in this species is enforced by the 18-electron rule, since CpFe(CO)2(η1-C3H5) is already an 18-electron complex, while an η3-allyl ligand would result in an electron count of 20 and violate the 18-electron rule. Such complexes can convert to the η3-allyl derivatives by dissociation of a neutral (two-electron) ligand L. For CpFe(CO)2(η1-C3H5), dissociation of L = CO occurs under photochemical conditions:[6]
- CpFe(CO)2(η1-C3H5) → CpFe(CO)(η3-C3H5) + CO
Benzyl complexes[]
Benzyl and allyl ligands often exhibit similar chemical properties. Benzyl commonly adopt either η1 or η3 bonding modes. The interconversion reactions parallel those of η1- or η3-allyl ligands:
- CpFe(CO)2(η1-CH2Ph) → CpFe(CO)(η3-CH2Ph) + CO
In all bonding modes, the benzylic carbon atom is more strongly attached to the metal as indicated by M-C bond distances, which differ by ca. 0.2 Å in η3-bonded complexes.[8] X-ray crystallography demonstrate that the benzyl ligands in tetrabenzylzirconium are highly flexible. One polymorph features four η2-benzyl ligands, whereas another polymorph has two η1- and two η2-benzyl ligands.[7]
Applications[]
In terms of applications, a popular allyl complex is allyl palladium chloride.[9] Allyl ligands are susceptible to nucleophilic addition, which can be useful in organic synthesis.[10]
References[]
- ^ a b Kevin D. John; Judith L. Eglin; Kenneth V. Salazar; R. Thomas Baker; Alfred P. Sattelberger (2014). Tris(Allyl)Iridium and -Rhodium. Inorganic Syntheses. 36. p. 165. doi:10.1002/9781118744994.ch32. ISBN 9781118744994.
- ^ Martin F. Semmelhack and Paul M. Helquist (1988). "Reaction of Aryl Halides with π-Allylnickel Halides: Methallylbenzene". Organic Syntheses.; Collective Volume, 6, p. 722
- ^ Craig R. Smith, Aibin Zhang, Daniel J. Mans, T. V. Rajanbabu (2008). "(R)-3-methyl-3-phenyl-1-pentene Via Catalytic Asymmetric Hydrovinylation". Org. Synth. 85: 248–266. doi:10.15227/orgsyn.085.0248. PMC 2723857. PMID 19672483.CS1 maint: uses authors parameter (link)
- ^ Putnik, Charles F.; Welter, James J.; Stucky, Galen D.; d'Aniello, M. J.; Sosinsky, B. A.; Kirner, J. F.; Muetterties, E. L. (1978). "Metal clusters in catalysis. 15. A Structural and Chemical Study of a Dinuclear Metal Complex, Hexacarbonylbis(.eta.3-2-propenyl)diiron(Fe-Fe)". Journal of the American Chemical Society. 100 (13): 4107–4116. doi:10.1021/ja00481a020.
- ^ Hirano, Masafumi; Sakate, Yumiko; Komine, Nobuyuki; Komiya, Sanshiro; Wang, Xian-qi; Bennett, Martin A. (2011). "Stoichiometric Regio- and Stereoselective Oxidative Coupling Reactions of Conjugated Dienes with Ruthenium(0). A Mechanistic Insight into the Origin of Selectivity". Organometallics. 30 (4): 768–777. doi:10.1021/om100956f.
- ^ Fish, R. W.; Giering, W. P.; Marten, D.; Rosenblum, M. (1976-01-27). "Thermal and photochemical interconversions of isomeric monocarbonyl η5-Cyclopentadienyl(η3-allyl)iron complexes". Journal of Organometallic Chemistry. 105 (1): 101–118. doi:10.1016/S0022-328X(00)91977-6. ISSN 0022-328X.
- ^ a b Rong, Yi; Al-Harbi, Ahmed; Parkin, Gerard (2012). "Highly Variable Zr–CH2–Ph Bond Angles in Tetrabenzylzirconium: Analysis of Benzyl Ligand Coordination Modes". Organometallics. 31pages=8208–8217 (23): 8208–8217. doi:10.1021/om300820b.
- ^ Trost, Barry M.; Czabaniuk, Lara C. (2014). "Structure and Reactivity of Late Transition Metal η3-Benzyl Complexes". Angew. Chem. Int. Ed. 53 (11): 2826–2851. doi:10.1002/anie.201305972. PMID 24554487.
- ^ Tatsuno, Y.; Yoshida, T.; Otsuka, S. "(η3-allyl)palladium(II) Complexes" Inorganic Syntheses, 1990, volume 28, pages 342-345. ISBN 0-471-52619-3
- ^ Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 189138953X
- Organometallic chemistry
- Transition metals
- Allyl complexes
- Coordination chemistry