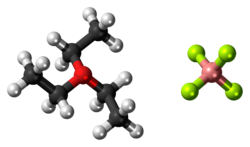
Triethyloxonium tetrafluoroborate
| |
| |
| Names | |
|---|---|
| IUPAC name
Triethyloxonium tetrafluoroborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3598090 | |
| ChemSpider | |
| ECHA InfoCard | 100.006.096 |
PubChem CID
|
|
| UNII | |
| UN number | 3261 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H15BF4O | |
| Molar mass | 189.99 g·mol−1 |
| Melting point | 91 to 92 °C (196 to 198 °F; 364 to 365 K) |
| Reacts | |
| Hazards[1] | |
| GHS labelling: | |

| |
Signal word
|
Danger |
| H314 | |
| P260, P280, P303+P361+P353, P304+P340+P310, P305+P351+P338, P310, P363 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triethyloxonium tetrafluoroborate is the organic oxonium compound with the formula [(CH3CH2)3O]BF4. It is often called Meerwein's reagent or Meerwein's salt after its discoverer Hans Meerwein.[2][3] Also well known and commercially available is the related trimethyloxonium tetrafluoroborate. The compounds are white solids that dissolve in polar organic solvents. They are strong alkylating agents. Aside from the BF−
4 salt, many related derivatives are available.[4]
Synthesis and reactivity[]
Triethyloxonium tetrafluoroborate is prepared from boron trifluoride, diethyl ether and epichlorohydrin:[5]
- 4 Et2O·BF3 + 2 Et2O + 3 C2H3(O)CH2Cl → 3 Et3O+BF−
4 + B[(OCH(CH2Cl)CH2OEt]3
The trimethyloxonium salt is available from dimethyl ether via an analogous route.[6] These salts do not have long shelf-lives at room temperature. They degrade by hydrolysis:
- [(CH3CH2)3O]+BF−
4 + H2O → (CH3CH2)2O + CH3CH2OH + HBF4
The propensity of trialkyloxonium salts for alkyl-exchange can be advantageous. For example, trimethyloxonium tetrafluoroborate, which reacts sluggishly due to it low solubility in most compatible solvents may be converted in situ to higher alkyl/more soluble oxoniums, thereby accelerating alkylation reactions.[7]
Structure[]
The structure of triethyloxonium tetrafluoroborate has not been characterized by X-ray crystallography, but the structure of triethyloxonium hexafluorophosphate has been examined. The measurements confirm that the cation is pyramidal with C-O-C angles in the range 109.4°–115.5°. The average C–O distance is 1.49 Å.[8]
Safety[]
Triethyloxonium tetrafluoroborate is a strong alkylating agent, although the hazards are diminished because it is non-volatile. It releases strong acid upon contact with water. The properties of the methyl derivative are similar.
References[]
- ^ "Triethyloxonium tetrafluoroborate". Sigma Aldrich.
- ^ H. Meerwein; G. Hinz; P. Hofmann; E. Kroning & E. Pfeil (1937). "Über Tertiäre Oxoniumsalze, I". Journal für Praktische Chemie. 147 (10–12): 257. doi:10.1002/prac.19371471001.
- ^ H. Meerwein; E. Bettenberg; H. Gold; E. Pfeil & G. Willfang (1940). "Über Tertiäre Oxoniumsalze, II". Journal für Praktische Chemie. 154 (3–5): 83. doi:10.1002/prac.19391540305.
- ^ Hartwig Perst, Dave G. Seapy "Triethyloxonium Tetrafluoroborate" in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, New York, 2008. doi:10.1002/047084289X.rt223.pub2. Article Online Posting Date: March 14, 2008
- ^ H. Meerwein (1973). "Triethyloxonium fluoroborate". Organic Syntheses.; Collective Volume, 5, p. 1080
- ^ T. J. Curphey (1988). "Trimethyloxonium tetrafluoroborate". Organic Syntheses.; Collective Volume, 6, p. 1019
- ^ Vartak A.P. & Crooks P.A. (2009). "A Scalable Enantioselective synthesis of the alpha2-adrenergic Agonist, Lofexidine". Org. Process Res. Dev. 13 (3): 415–419. doi:10.1021/op8002689.
- ^ Watkins, Michael I.; Ip, Wai Man; Olah, George A.; Bau, Robert (1982). "Structure of Oxonium Ions: An X-Ray Crystallographic Study of Triethyloxonium Hexafluorophosphate and Triphenyloxonium Tetraphenylborate". Journal of the American Chemical Society. 104 (9): 2365–2372. doi:10.1021/ja00373a006.
- Reagents for organic chemistry
- Tetrafluoroborates
- Ethylating agents
- Oxonium compounds