Vanilloids
The vanilloids are compounds which possess a vanillyl group. They include vanillyl alcohol, vanillin, vanillic acid, acetovanillon, vanillylmandelic acid, homovanillic acid, capsaicin, etc. Isomers are the isovanilloids.
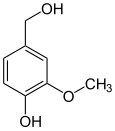
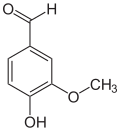

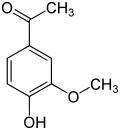
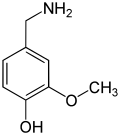
vanillyl alcohol vanillin vanillic acid acetovanillon Vanillylamine
A number of vanilloids, most notably capsaicin, bind to the transient receptor potential vanilloid type 1 (TRPV1) receptor, an ion channel which naturally responds to noxious stimuli such as high temperatures and acidic pH.[1] This action is responsible for the burning sensation experienced after eating spicy peppers.
Outside the food industry vanilloids such as nonivamide are used commercially in pepper spray formulations.
Other vanilloids which act at TRPV1 include resiniferatoxin and .
References[]
- ^ Pingle, SC; Matta, JA; Ahern, GP (2007). "Capsaicin receptor: TRPV1 a promiscuous TRP channel". Handbook of Experimental Pharmacology. 179 (179): 155–171. doi:10.1007/978-3-540-34891-7_9. ISBN 978-3-540-34889-4. PMID 17217056. Cite journal requires
|journal=(help) - ^ Carlson, Neil R.; Birkett, Melissa A. (2017). Physiology of Behavior (12 ed.). Pearson. p. 212. ISBN 9780134320823.
Literature[]
- Lee, Jeewoo; Uk Kang, Sang; Yeon Kim, Su; Eun Kim, Sung; Joon Jo, Yeong; Kim, Sunghoon (2001). "Vanilloid and Isovanilloid Analogues as Inhibitors of Methionyl-tRNA and Isoleucyl-tRNA Synthetases" (PDF). Bioorganic & Medicinal Chemistry Letters. 11 (8): 965–968. doi:10.1016/S0960-894X(01)00096-8. PMID 11327601.[permanent dead link]
Categories:
- Vanilloids
- Phenols
- Phenol ethers




