Xenon oxytetrafluoride
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| XeOF4 | |
| Molar mass | 223.23 g/mol |
| Appearance | colorless liquid |
| Density | 3.17 g/cm3, liquid |
| Melting point | −46.2 °C (−51.2 °F; 227.0 K) |
| Reacts with water | |
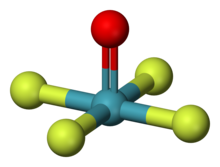
| Structure | |
| square pyramidal[1][2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xenon oxytetrafluoride (XeOF4) is an inorganic chemical compound. It is a colorless stable liquid[3][2] with a melting point of −46.2 °C[4] that can be synthesized by partial hydrolysis of XeF
6, or the reaction of XeF
6 with silica[3] or NaNO
3:[5]
- NaNO
3 + XeF
6 → NaF + XeOF
4 + FNO
2
A high-yield synthesis proceeds by the reaction of XeF
6 with POF
3 at −196 °C.[6]
As are most xenon oxides, it is extremely reactive and unstable, and hydrolyses in water to give dangerously hazardous and corrosive products, including hydrogen fluoride:
- 2 XeOF4 + 4 H2O → 2 Xe + 8 HF + 3 O2
In addition, some ozone and fluorine are also formed. This reaction is extremely dangerous, and xenon oxytetrafluoride should therefore be kept away from any trace of water or water vapour under all conditions.
Reactions[]
XeOF4 reacts with H2O in a following steps:
- XeOF4 + H2O → XeO2F2 + 2 HF
- XeO2F2 + H2O → XeO3 + 2 HF
The XeO3 formed is a dangerous explosive, decomposing explosively to Xe and O2:
- 2 XeO3 → 2 Xe + 3 O2
In its liquid form, XeOF
4 exhibits amphoteric behaviour, forming complexes with both strong Lewis bases like CsF and strong Lewis acids like SbF
5.[7] It forms a 1:1 adduct with XeF
2, isostructural with XeF
2·IF
5,[8] as well as various heavy alkali metal fluorides.[4]
The reaction of XeOF
4 with XeO
3 provides a convenient synthesis route for .[9]
External links[]
References[]
- ^ Martins, Joseph; E. Bright Wilson Jr. (1964). "Microwave Spectrum of Xenon Oxytetrafluoride". J. Chem. Phys. 41 (570): 570–571. Bibcode:1964JChPh..41..570M. doi:10.1063/1.1725910.
- ^ a b D. F. Smith (24 May 1963). "Xenon Oxyfluoride". Science. 140 (3569): 899–900. Bibcode:1963Sci...140..899S. doi:10.1126/science.140.3569.899. PMID 17810680. S2CID 42752536.
- ^ a b Ibers, James A. (Oct 1965). "Molecular Structure". Annual Review of Physical Chemistry. 16: 375–396. Bibcode:1965ARPC...16..375I. doi:10.1146/annurev.pc.16.100165.002111.
- ^ a b Selig, Henry (Feb 1, 1966). "Complexes of Xenon Oxide Tetrafluoride". Inorg. Chem. 5 (2): 183–186. doi:10.1021/ic50036a004.CS1 maint: date and year (link)
- ^ Christe, Karl O.; Wilson, William W. (Apr 1988). "Convenient synthesis of xenon oxide tetrafluoride". Inorg. Chem. 27 (7): 1296–1297. doi:10.1021/ic00280a043.CS1 maint: date and year (link)
- ^ Nielsen, Jon B.; Kinkead, Scott A.; Eller, P. Gary (Sep 1, 1990). "A New Synthesis of Xenon Oxytetrafluoride, XeOF
4". Inorg. Chem. 29 (18): 3621–3622. doi:10.1021/ic00343a063.CS1 maint: date and year (link) - ^ D.Martin-Rovet; C.Angelié; M.Cauchetier; G.J.Schrobilgen (Sep 1982). "Various aspects of the reactivity of the xenon(VI) oxyfluoride: XeOF
4". Journal of Fluorine Chemistry. 21 (1): 10. doi:10.1016/S0022-1139(00)85330-0. - ^ N. Bartlett; M. Wechsberg (Oct 1971). "The Xenon Difluoride Complexes XeF
2 · XeOF
4; XeF
2 · XeF
6 · AsF
5 and XeF
2 · 2 XeF
6 · 2 AsF
5 and Their Relevance to Bond Polarity and Fluoride Ion Donor Ability of XeF
2 and XeF
6". Z. Anorg. Allg. Chem. 385 (1). doi:10.1002/zaac.19713850103. - ^ Huston, John L. (Sep 1967). "Xenon dioxide difluoride: isolation and some properties". J. Phys. Chem. 71 (10): 3339–3341. doi:10.1021/j100869a035.CS1 maint: date and year (link)
- Xenon(VI) compounds
- Oxohalides
- Inorganic compound stubs