Xylindein
| |

| |
| Names | |
|---|---|
| IUPAC name
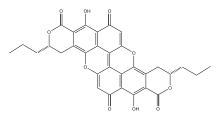
(7S,20S)-11,24-dihydroxy-7,20-dipropyl-8,16,21,30-tetraoxaoctacyclo[15.11.1.14,28.02,15.03,12.05,10.018,23.025,29]triaconta-1,3,5(10),11,14,17(29),18(23),24,27-nonaene-9,13,22,26-tetrone
| |
| Preferred IUPAC name
(3S,11S)-8,16-Dihydroxy-3,11-dipropyl-3,4,11,12-tetrahydro-1H,7H-pyrano[4,3-h]pyrano[4′,3′:5,6]xantheno[2,1,9,8-klmna]xanthene-1,7,9,15-tetrone | |
| Other names
Xylindene
(3S,11S)-3,4,11,12-Tetrahydro-8,16-dihydroxy-3,11-dipropyl-1H,7H-dipyrano[4,3-a:4',3'-j]-peri-xanthenoxanthene-1,7,9,15-tetrone peri-xanthenoxanthene-2,8-dicarboxy-lic acid 4,10-dihydro-3,9-dihydroxy-1,7-bis(2 S-hydroxy-pentyl)-4,10-dioxo-di δ-lactone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| Properties | |
| C32H24O10 | |
| Molar mass | 568.534 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xylindein is a quinone pigment, a dimeric naphthoquinone derivative. It is produced by fungi from genus Chlorociboria. This pigment causes green staining of wood infected by the fungi.
Etymology[]
This pigment was firstly extracted in 1868 by from wood and resembled indigo, so he called it xylindéine. Combination of xyl- (wood) and indé (indigo) + -ine.[1][2]
References[]
- ^ Xylindein at Merriam-Webster dictionary
- ^ Thenard, Paul; Alphonse Rommier (1868). "Sur un nouvelle matière colorante appelée xylindeine et extraite de certains bois morts". Comptes rendus hebdomadaires des séances de l'Académie des Sciences (in French). Paris. 66: 108–109. ISSN 0001-4036.
- Saikawa, Yoko; Watanabe, Takashi; Hashimoto, Kimiko; Nakata, Masaya (October 2000). "Absolute configuration and tautomeric structure of xylindein, a blue-green pigment of Chlorociboria species". Phytochemistry. Elsevier. 55 (3): 237–240. doi:10.1016/S0031-9422(00)00282-X. PMID 11142849.
- Donner, Christopher; Cuzzupe, Anthony; Falzon, Cheryl; Gill, Melvyn (April 2012). "Investigations towards the synthesis of xylindein, a blue-green pigment from the fungus Chlorociboria aeruginosa". Tetrahedron. Elsevier. 68 (13): 2799–2805. doi:10.1016/j.tet.2012.02.009.
- Robinson, Sara; Tudor, Daniela; Snider, Hilary; Cooper, Paul (March 2012). "Stimulating growth and xylindein production of Chlorociboria aeruginascens in agar-based systems". AMB Express. Springer. 2: 15. doi:10.1186/2191-0855-2-15. PMC 3350399. PMID 22409931.
- Ostroverkhova, Oksana; Robinson, Sara; Gutierrez, Sarath Vega; Schenck, Jonathan Van; Giesbers, Gregory (2018). "Fungi-Derived Pigments for Sustainable Organic (Opto)Electronics". MRS Advances. 3 (59): 3459–3464. doi:10.1557/adv.2018.446. ISSN 2059-8521.
External links[]
 Media related to Xylindein at Wikimedia Commons
Media related to Xylindein at Wikimedia Commons
Categories:
- Biological pigments
- Heterocyclic compounds with 7 or more rings
- Natural phenol dimers
- Dihydroisocoumarins
- Pyrans
- Hydroxyarenes
- 3-hydroxypropenals within hydroxyquinones
- Polyenes
- Naphthoquinones