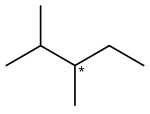
2,3-Dimethylpentane
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dimethylpentane | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.437 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| UN number | 1206 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H16 | |
| Molar mass | 100.205 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 0.7076 g/mL (25 °C), 0.6413 (80 °C), 0.7380 (25 °C, 45 MPa), 0.6891 (80 °C, 45 MPa) (racemic)[1] |
| Boiling point | 89.7 °C (racemic)[2][3][4] |
| Vapor pressure | 2.35 psi (37.7 °C)[5] |
| Viscosity | 0.356 mPa s (30 °C), 0.232 (80 °C), 0.624 (30 °C, 60 MPa) (racemic)[1] |
| Thermochemistry | |
Heat capacity (C)
|
34.308 cal/K/mol (−189 °C), 51.647 (20 °C), 58.735 (86.6 °C) (racemic)[6] |
Std molar
entropy (S |
71.02 cal/K/mol (25 °C) (racemic)[6] |
| Hazards | |
| GHS labelling: | |
   
| |
Signal word
|
Danger |
| H225, H304, H315, H335, H336, H410 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P301+P310, P302+P352, P303+P361+P353, P304+P340, P312, P321, P331, P332+P313, P362, P370+P378, P391, P403+P233, P403+P235, P405, P501 | |
| Flash point | −7 °C[5] |
| 337 °C (639 °F; 610 K)[5] | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,3-Dimethylpentane is an organic compound of carbon and hydrogen with formula C
7H
16, more precisely CH
3–CH(CH
3)–CH(CH
3)–CH
2–CH
3: a molecule of pentane with methyl groups –CH
3 replacing hydrogen atoms on carbon atoms 2 and 3. It is an alkane ("paraffin" in older nomenclature), a fully saturated hydrocarbon; specifically, one of the isomers of heptane.
Like typical alkanes, it is a colorless flammable compound; under common ambient conditions, it is a mobile liquid, less dense than water.[1]
2,3-Dimethylpentane is notable for being one of the two simplest alkanes with optical (enantiomeric) isomerism. The optical center is the middle carbon of the pentane backbone, which is connected to one hydrogen atom, one methyl group, one ethyl group –C
2H
5, and one isopropyl group –CH(CH
3)
2. The two enantiomers are denoted (3R)-2,3-dimethylpentane and (3S)-2,3-dimethylpentane. (The other simplest chiral alkane is its structural isomer 3-methylhexane.)
Properties[]
Most properties listed in the literature refer to the racemic compound (an equimolar mixture of the two optical isomers).
The boiling point of 89.7 °C is 0.3 °C higher than the value of 89.4 °C predicted by Wiener's formula, based on the structure of the molecule and the boiling point of n-heptane.[2][3]
The speed of sound at 3 MHz is 1149.5 m/s at 20 °C and 889.5 m/s at 80 °C.[7][8][9]
The racemic mixture has a glass transition temperature of about 123 K (−150 °C), but reportedly it does not crystallize—a fact that has been claimed to be a characteristic of high-purity optically active alkanes.[4][6][10]
Preparation[]
2,3-Dimethylpentane is practically absent in the synthetic fuel produced from hydrogen and carbon monoxide by the Fischer–Tropsch process.[11]
The pure compound can be prepared by reacting the Grignard reagent C
4H
9–MgBr with acetone to form , then dehydrating this alcohol to form , and hydrogenating this product.[4]
The isomer is present at about 2.4% by weight in the hydrocarbon mixture obtained by the condensation of methanol at 200 °C with a zinc iodide catalyst (the main component of the mixture being the isomer 2,2,3-trimethylbutane, obtained at almost 50% yield).[12]
See also[]
- trans-1,2-Dimethylcyclopropane (C
5H
10), the simplest chiral cycloalkane
References[]
- ^ a b c Alfonso S. Pensado, María J. P. Comuñas, Luis Lugo, and Josefa Fernández (2005): "Experimental Dynamic Viscosities of 2,3-Dimethylpentane up to 60 MPa and from (303.15 to 353.15) K Using a Rolling-Ball Viscometer". Journal of Chemical & Engineering Data, volume 50, issue 3, pages 849–855. doi:10.1021/je049662k
- ^ a b Pentane, 2,3-dimethyl-. NIST Chemistry WebBook, SRD 69. Accessed on 2018-11-06.
- ^ a b Harry Wiener (1947): "Structural Determination of Paraffin Boiling Points". Journal of the American Chemical Society, volume 69, issue 1, pages 17–20. doi:10.1021/ja01193a005
- ^ a b c Graham Edgar, George Calingaert, and R. E. Marker (1929): The preparation and properties of the isomeric heptanes. Part I. Preparation". Journal of the American Chemical Society, volume 51, issue 5, pages 1483–1491. doi:10.1021/ja01380a027. Density 0.6952, refractive index 1.39201 at 20 °C.
- ^ a b c Sigma Aldrich: 2,3-Dimethylpentane catalog entry. Accessed on 2018-11-06.
- ^ a b c H. L. Finke, J. F. Messerly, D. R. Douslin (1976): "Low-temperature thermal quantities for five alkyl-substituted pentanes". The Journal of Chemical Thermodynamics, volume 8, issue 10, pages 965-983. doi:10.1016/0021-9614(76)90113-0
- ^ F. Plantier and J. L. Daridon (2005): "Speed of Sound of 2-Methylpentane, 2,3-Dimethylpentane, and 2,2,4-Trimethylpentane from (293.15 to 373.15) K and up to 150 MPa". Journal of Chemical & Engineering Data, volume 50, issue 6, pages 2077–2081. doi:10.1021/je0502849. Note: density values 0.6943 g/mL at 20 °C and 0.6377 at 80°C differ significantly from those of Pensado et al. Also gives data at 100 kPA up to 100 °C which is above the boiling point.
- ^ Egbert B. Freyer, J. C. Hubbard, and Donald H. Andrews (1929): "Sonic studies of the physical properties of liquids. I. The sonic interferometer. The velocity of sound in some organic liquids and their compressibilities". Journal of the American Chemical Society, volume 51, issue 3, pages 759–770. doi:10.1021/ja01378a014 Note: gives speed of ~400 Hz sound as 1148.5 at 20 °C and 1039 m/s at 45 °C, and density 0.6942 g/mL at 20 °C.
- ^ J. L. E. Chevalier, P. J. Petrino, and Y. H. Gaston-Bonhomme (1990): "Viscosity and density of some aliphatic, cyclic, and aromatic hydrocarbons binary liquid mixtures". Journal of Chemical & Engineering Data, volume 35, issue 2, pages 206–212. doi: 10.1021/je00060a034. Gives kinematic viscosity 0.5513 m2/s and density 0.69026 g/mL at 25 °C.
- ^ John M. Smith, John M. Simmie, Henry J. Curran (2005): "Autoignition of heptanes; experiments and modeling". International Journal of Chemical Kinetics, volume 37, issue 12, pages 728-736. doi:10.1002/kin.20120
- ^ R. A. Friedel, and R. B. Anderson (1950): "Composition of Synthetic Liquid Fuels. I. Product Distribution and Analysis of C5—C8 Paraffin Isomers from Cobalt Catalyst". Journal of the American Chemical Society, volume 72, issue 3, pages 1212–1215. doi:10.1021/ja01159a039
- ^ Leo Kim, Milton M. Wald, Stanley G. Brandenberger (1978): "One-step catalytic synthesis of 2,2,3-trimethylbutane from methanol." Journal of Organic Chemistry, volume 43, issue 17, pages 3432-3433. doi:10.1021/jo00411a053
- Alkanes