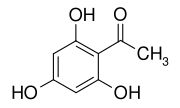
2,4,6-Trihydroxyacetophenone
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(2,4,6-Trihydroxyphenyl)ethan-1-one | |
| Other names
1-(2,4,6-Trihydroxyphenyl)ethanone
2-Acetylphloroglucinol THAP Phloroacetophenone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.870 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O4 | |
| Molar mass | 168.148 g·mol−1 |
| Melting point | 219 to 221 °C (426 to 430 °F; 492 to 494 K) |
| Hazards | |
| GHS labelling: | |

| |
Signal word
|
Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,4,6-Trihydroxyacetophenone (THAP) is a chemical compound that is a derivative of phloroglucinol.
In an animal model, THAP was reported to enhance cholesterol 7 alpha-hydroxylase (CYP7A1) activity.[1]
THAP is also used as a matrix in matrix-assisted laser desorption/ionization (MALDI) for the analysis of acidic glycans and glycopeptides in negative ion mode.
Derivatives[]
THAP is a chemical precursor that can be used to form part of the backbone of 5,7-dihydroxyflavones like ,[2] apigenin, luteolin, diosmetin, naringenin, and hesperetin.
See also[]
References[]
- ^ Charoenteeraboon, Juree; Nithipatikom, Kasem; Campbell, William B.; Piyachaturawat, Pawinee; Wilairat, Prapon; Rongnoparut, Pornpimol (2005). "Induction of human cholesterol 7α-hydroxylase in HepG2 cells by 2,4,6-trihydroxyacetophenone". European Journal of Pharmacology. 515 (1–3): 43–46. doi:10.1016/j.ejphar.2005.03.039. PMID 15896733.
- ^ Bruder, Marjorie; Haseler, Paul L.; Muscarella, Marina; Lewis, William; Moody, Christopher J. (2010). "Synthesis of the Oxepinochromone Natural Products Ptaeroxylin (Desoxykarenin), Ptaeroxylinol, and Eranthin". The Journal of Organic Chemistry. 75 (2): 353–358. doi:10.1021/jo902117e. ISSN 0022-3263.
Categories:
- Phloroglucinols
- Aromatic ketones
- 3-hydroxypropenals