Apigenin
| |

| |
| Names | |
|---|---|
| IUPAC name
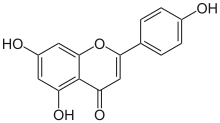
4′,5,7-Trihydroxyflavone
| |
| Preferred IUPAC name
5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Apigenine; Chamomile; Apigenol; Spigenin; Versulin; C.I. Natural Yellow 1
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.540 |
IUPHAR/BPS
|
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C15H10O5 |
| Molar mass | 270.240 g·mol−1 |
| Appearance | Yellow crystalline solid |
| Melting point | 345 to 350 °C (653 to 662 °F; 618 to 623 K) |
| UV-vis (λmax) | 267, 296sh, 336 nm in methanol[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.
Sources in nature[]
Apigenin is found in many fruits and vegetables, but parsley, celery, celeriac, and chamomile tea are the most common sources.[3] Apigenin is particularly abundant in the flowers of chamomile plants, constituting 68% of total flavonoids.[4] Dried parsley can contain about 45 mg/gram and dried chamomile flower about 3-5 mg/gram apigenin.[5] The apigenin content of fresh parsley is reportedly 215.5 mg/100 grams, which is much higher than the next highest food source, green celery hearts providing 19.1 mg/100 grams.[6]
Biosynthesis[]

Apigenin is biosynthetically derived from the general phenylpropanoid pathway and the flavone synthesis pathway.[7] The phenylpropanoid pathway starts from the aromatic amino acids L-phenylalanine or L-tyrosine, both products of the Shikimate pathway.[8] When starting from L-phenylalanine, first the amino acid is non-oxidatively deaminated by phenylalanine ammonia lyase (PAL) to make cinnamate, followed by oxidation at the para position by cinnamate 4-hydroxylase (C4H) to produce p-coumarate. As L-tyrosine is already oxidized at the para position, it skips this oxidation and is simply deaminated by tyrosine ammonia lyase (TAL) to arrive at p-coumarate.[9] To complete the general phenylpropanoid pathway, 4-coumarate CoA ligase (4CL) substitutes coenzyme A (CoA) at the carboxy group of p-coumarate. Entering the flavone synthesis pathway, the type III polyketide synthase enzyme chalcone synthase (CHS) uses consecutive condensations of three equivalents of malonyl CoA followed by aromatization to convert p-coumaroyl-CoA to chalcone.[10] Chalcone isomerase (CHI) then isomerizes the product to close the pyrone ring to make naringenin. Finally, a flavanone synthase (FNS) enzyme oxidizes naringenin to apigenin.[11] Two types of FNS have previously been described; FNS I, a soluble enzyme that uses 2-oxogluturate, Fe2+, and ascorbate as cofactors and FNS II, a membrane bound, NADPH dependent cytochrome p450 monooxygenase.[12]
Glycosides[]
The naturally occurring glycosides formed by the combination of apigenin with sugars include:
- Apiin (apigenin 7-O-apioglucoside), isolated from parsley[13] and celery
- Apigetrin (apigenin 7-glucoside), found in dandelion coffee
- Vitexin (apigenin 8-C-glucoside)
- Isovitexin (apigenin 6-C-glucosid)
- Rhoifolin (apigenin 7-O-neohesperidoside)
- (apigenin 6-C-glucoside 8-C-arabinoside)
See also[]
- Amentoflavone
References[]
- ^ Merck Index, 11th Edition, 763.
- ^ The Systematic Identification of Flavonoids. Mabry et al, 1970, page 81
- ^ The compound in the Mediterranean diet that makes cancer cells 'mortal' Emily Caldwell, Medical Express, May 20, 2013.
- ^ Venigalla M, Gyengesi E, Münch G (August 2015). "Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease". Neural Regeneration Research. 10 (8): 1181–5. doi:10.4103/1673-5374.162686. PMC 4590215. PMID 26487830.
- ^ Shankar E, Goel A, Gupta K, Gupta S (2017). "Plant flavone apigenin: An emerging anticancer agent". . 3 (6): 423–446. doi:10.1007/s40495-017-0113-2. PMC 5791748. PMID 29399439.
- ^ Delage, PhD, Barbara (November 2015). "Flavonoids". Linus Pauling Institute, Oregon State University, Corvallis, Oregon. Retrieved 2021-01-26.
- ^ Forkmann, G. (January 1991). "Flavonoids as Flower Pigments: The Formation of the Natural Spectrum and its Extension by Genetic Engineering". Plant Breeding. 106 (1): 1–26. doi:10.1111/j.1439-0523.1991.tb00474.x. ISSN 0179-9541.
- ^ Herrmann KM (January 1995). "The shikimate pathway as an entry to aromatic secondary metabolism". Plant Physiology. 107 (1): 7–12. doi:10.1104/pp.107.1.7. PMC 161158. PMID 7870841.
- ^ Lee H, Kim BG, Kim M, Ahn JH (September 2015). "Biosynthesis of Two Flavones, Apigenin and Genkwanin, in Escherichia coli". Journal of Microbiology and Biotechnology. 25 (9): 1442–8. doi:10.4014/jmb.1503.03011. PMID 25975614.
- ^ Austin MB, Noel JP (February 2003). "The chalcone synthase superfamily of type III polyketide synthases". Natural Product Reports. 20 (1): 79–110. CiteSeerX 10.1.1.131.8158. doi:10.1039/b100917f. PMID 12636085.
- ^ Martens S, Forkmann G, Matern U, Lukacin R (September 2001). "Cloning of parsley flavone synthase I". Phytochemistry. 58 (1): 43–6. doi:10.1016/S0031-9422(01)00191-1. PMID 11524111.
- ^ Leonard E, Yan Y, Lim KH, Koffas MA (December 2005). "Investigation of two distinct flavone synthases for plant-specific flavone biosynthesis in Saccharomyces cerevisiae". Applied and Environmental Microbiology. 71 (12): 8241–8. doi:10.1128/AEM.71.12.8241-8248.2005. PMC 1317445. PMID 16332809.
- ^ Meyer H, Bolarinwa A, Wolfram G, Linseisen J (2006). "Bioavailability of apigenin from apiin-rich parsley in humans". Annals of Nutrition & Metabolism. 50 (3): 167–72. doi:10.1159/000090736. PMID 16407641. S2CID 8223136.
- Aromatase inhibitors
- Delta-opioid receptor antagonists
- Flavones
- GABAA receptor positive allosteric modulators
- Resorcinols
- Kappa-opioid receptor antagonists
- Mu-opioid receptor antagonists
- NMDA receptor antagonists
- Phytoestrogens
- Progestogens
- Vinylogous carboxylic acids