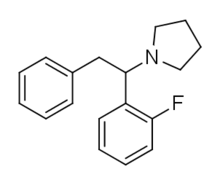
Fluorolintane
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C18H20FN |
| Molar mass | 269.363 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fluorolintane (also known as 2-FPPP and 2-F-DPPy) is a dissociative anesthetic drug that has been sold online as a designer drug.[1][2][3][4]
Fluorolintane and related diarylethylamines are antagonists of the NMDA receptor[5] and have been studied in vitro as potential treatments for neurotoxic injury,[6] depression[7] and as sympathomimetic.[8]
See also[]
- AD-1211
- Diphenidine
- Ephenidine
- Lanicemine
- Methoxphenidine (MXP)
- MT-45
- Prolintane
- Remacemide
References[]
- ^ Wallach J, Kavanagh PV, McLaughlin G, Morris N, Power JD, Elliott SP, et al. (May 2015). "Preparation and characterization of the 'research chemical' diphenidine, its pyrrolidine analogue, and their 2,2-diphenylethyl isomers" (PDF). Drug Testing and Analysis. 7 (5): 358–67. doi:10.1002/dta.1689. PMID 25044512.
- ^ "Analytical Report - Fluorolintane" (PDF). Nacionalni forenzični laboratorij (NFL). January 2016.
- ^ Wallach, Jason; Colestock, Tristan; Agramunt, Julià; Claydon, Matt D. B.; Dybek, Michael; Filemban, Nadine; Chatha, Muhammad; Halberstadt, Adam L.; Brandt, Simon D.; Lodge, David; Bortolotto, Zuner A.; Adejare, Adeboye (August 2019). "Pharmacological characterizations of the 'legal high' fluorolintane and isomers". European Journal of Pharmacology. 857: 172427. doi:10.1016/j.ejphar.2019.172427. ISSN 0014-2999. PMC 6899220. PMID 31152702.
- ^ Dybek, Michael; Wallach, Jason; Kavanagh, Pierce V.; Colestock, Tristan; Filemban, Nadine; Dowling, Geraldine; Westphal, Folker; Elliott, Simon P.; Adejare, Adeboye; Brandt, Simon D. (August 2019). "Syntheses and analytical characterizations of the research chemical 1-[1-(2-fluorophenyl)-2-phenylethyl]pyrrolidine (fluorolintane) and five of its isomers". Drug Testing and Analysis. 11 (8): 1144–1161. doi:10.1002/dta.2608. ISSN 1942-7611. PMID 31033229.
- ^ Berger ML, Schweifer A, Rebernik P, Hammerschmidt F (May 2009). "NMDA receptor affinities of 1,2-diphenylethylamine and 1-(1,2-diphenylethyl)piperidine enantiomers and of related compounds". Bioorganic & Medicinal Chemistry. 17 (9): 3456–62. doi:10.1016/j.bmc.2009.03.025. PMID 19345586.
- ^ Gray NM, Cheng BK (1994). "Patent EP 0346791 B1 - 1,2-diarylethylamines for treatment of neurotoxic injury".
- ^ Aspergren BD, Heinzelman RV (1963). "Patent US 3083139 A - Therapeutic 1-(1, 2-diphenylethyl) pyrrolidine for the management of depression".
- ^ Heinzelman RV, Aspergren BD (July 1953). "Compounds Containing the Pyrrolidine Ring. Analogs of Sympathomimetic Amines". Journal of the American Chemical Society. 75 (14): 3409–3413. doi:10.1021/ja01110a033.
Categories:
- Designer drugs
- Dissociative drugs
- NMDA receptor antagonists
- Diarylethylamines
- Fluoroarenes
- Pyrrolidines
- Nervous system drug stubs