Not to be confused with
α-PCyP .
Rolicyclidine ATC code Legal status
AU :S9 (Prohibited substance) CA Schedule III DE Anlage I (Authorized scientific use only) UK :Class A US :Schedule I
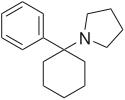
1-(1-phenylcyclohexyl)pyrrolidine
CAS Number PubChem CID DrugBank ChemSpider UNII ChEBI CompTox Dashboard (EPA ) Formula C 16 H 23 N Molar mass −1 3D model (JSmol )
c1ccccc1C3(N2CCCC2)CCCCC3
InChI=1S/C16H23N/c1-3-9-15(10-4-1)16(11-5-2-6-12-16)17-13-7-8-14-17/h1,3-4,9-10H,2,5-8,11-14H2
Y Key:FYOWWXMGDATDQY-UHFFFAOYSA-N
Y
Rolicyclidine (PCPy ) is a dissociative anesthetic drug with hallucinogenic and sedative effects. It is similar in effects to phencyclidine but is slightly less potent and has less stimulant effects[1] barbiturate , but with additional PCP-like dissociative, anaesthetic and hallucinogenic effects.[2]
See also [ ] PCP Arylcyclohexylamine Picilorex α-PHP References [ ]
^ Kalir A, Edery H, Pelah Z, Balderman D, Porath G (May 1969). "1-Phenycycloalkylamine derivatives. II. Synthesis and pharmacological activity". Journal of Medicinal Chemistry . 12 (3): 473–7. doi :10.1021/jm00303a030 . PMID 4977945 . ^ DEA Microgram Bulletin, 8, 143, 1975
General anesthetics (N01A )
Inhalational
Chloroethane (ethyl chloride) ‡ Chloroform ‡ Cyclopropane ‡ Desflurane Diethyl ether ‡ Enflurane Ethylene ‡ Fluroxene ‡ Halothane # Isoflurane # Methoxyflurane Methoxypropane ‡ Nitrous oxide # Sevoflurane Trichloroethylene ‡ Vinyl ether ‡ Xenon Injection
Phenols Barbiturates
Hexobarbital Methohexital Narcobarbital Thiopental # Thiotetrabarbital Opioids
Morphine # Oxycodone Anileridine ‡ Embutramide ‡ Fentanyl # Alfentanil Phenoperidine Remifentanil ÷Sufentanil Arylcyclohexylamines Neuroactive steroids
Alfadolone Alfaxalone Hydroxydione Others
# WHO-EM ‡ Withdrawn from marketClinical trials :
† Phase III § Never to phase III
Hallucinogens
Psychedelics (5-HT2A
Benzofurans
2C-B-FLY 2CBFly-NBOMe 5-MeO-BFE 5-MeO-DiBF Bromo-DragonFLY F-2 F-22 TFMFly Lyserg‐
1B-LSD 1cP-LSD 1P-ETH-LAD 1P-LSD 1V-LSD 2-Butyllysergamide 3-Pentyllysergamide AL-LAD ALD-52 BU-LAD Diallyllysergamide Dimethyllysergamide ECPLA Ergometrine ETH-LAD IP-LAD LAE-32 LPD-824 LSA LSD LSD-Pip LSH LSM-775 LSZ Methylergometrine MIPLA Methysergide MLD-41 PARGY-LAD PRO-LAD Phenethyl‐
Piperazines Tryptamines
alpha -alkyltryptamines
4,5-DHP-α-MT 5-MeO-α-ET 5-MeO-α-MT α-ET α-MT x -DALT
(Daltacetin) 4-AcO-DALT 5-MeO-DALT DALT x -DET
(Ethacetin) 4-AcO-DET (Ethocin) 4-HO-DET 5-MeO-DET (T-9) DET (Ethocybin) 4-PO-DET x -DiPT
(Ipracetin) 4-AcO-DiPT (Iprocin) 4-HO-DiPT 5-MeO-DiPT DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4-Propionyloxy-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT
(Deprocin) 4-HO-DPT 5-MeO-DPT (The Light) DPT Ibogaine-related
18-MAC 18-MC Coronaridine Ibogaine Ibogamine ME-18-MC Noribogaine Tabernanthine Voacangine x -MET
(Metocin) 4-HO-MET (Metocetin) 4-AcO-MET 5-MeO-MET MET x -MiPT
(Mipracetin) 4-AcO-MiPT (Miprocin) 4-HO-MiPT 5-Me-MiPT (Moxy) 5-MeO-MiPT MiPT Others
4-HO-DBT 4-HO-EPT 4-HO-McPT (Lucigenol) 4-HO-MPMI (Meprocin) 4-HO-MPT 5-MeO-EiPT 5-MeO-MALT 5-MeO-MPMI Aeruginascin Baeocystin DBT EiPT EPT MPT PiPT
Others
AL-38022A ALPHA Dimemebfe Efavirenz Glaucine Lorcaserin M-ALPHA RH-34 Also empathogens in general (e. g.: 5-APB , 5-MAPB , 6-APB and other substituted benzofurans ).
Dissociatives (NMDAR antagonists )
Arylcyclo‐
Ketamine-related PCP-related Others
Diarylethylamines Morphinans
Dextrallorphan Dextromethorphan Dextrorphan Racemethorphan Racemorphan Others
Deliriants (mAChR antagonists ) Others
Cannabinoids (CB1 agonists)
Natural
Salvinorin A THC (Dronabinol) THCV Synthetic
D2 agonists
Apomorphine Aporphine Bromocriptine Cabergoline Lisuride LSD Memantine Nuciferine Pergolide Phenethylamine Piribedil Pramipexole Ropinirole Rotigotine Salvinorin A Also indirect D2 agonists, such as dopamine reuptake inhibitors (cocaine , methylphenidate ), releasing agents (amphetamine , methamphetamine ), and precursors (levodopa ). GABAA
CI-966 Eszopiclone Ibotenic acid Muscimol (Amanita muscaria Zaleplon Zolpidem Zopiclone Inhalants (Mixed MOA )
Aliphatic hydrocarbons
Butane Gasoline Kerosene Propane Aromatic hydrocarbons
Ethers
Haloalkanes
Chlorofluorocarbons Chloroform κOR agonists
2-EMSB Alazocine Bremazocine Butorphan Butorphanol Cyclazocine Cyclorphan Cyprenorphine Diprenorphine Enadoline Herkinorin Heroin HZ-2 Ibogaine Ketazocine Levallorphan Levomethorphan Levorphanol LPK-26 Metazocine Morphine Nalbuphine Nalmefene Nalorphine Noribogaine Oxilorphan Pentazocine Phenazocine Proxorphan Racemethorphan Racemorphan Salvinorin A Spiradoline Tifluadom U-50488 U-69,593 Xorphanol Oneirogens
Calea zacatechichi Silene capensis Galantamine Others
Glaucine Isoaminile Noscapine Pukateine
D1 -like
Agonists
Benzazepines : 6-Br-APB Fenoldopam SKF-38,393 SKF-77,434 SKF-81,297 SKF-82,958 SKF-83,959 Trepipam Ergolines : Cabergoline CY-208,243 Dihydroergocryptine Lisuride Pergolide Terguride Dihydrexidine derivatives : A-77636 A-86929 Adrogolide (ABT-431, DAS-431) Dihydrexidine Dinapsoline Dinoxyline Doxanthrine Phenethylamines : Deoxyepinephrine (N-methyldopamine, epinine) Dopexamine Etilevodopa Ibopamine L -DOPA (levodopa)Melevodopa L -PhenylalanineL -TyrosineOthers : A-68930 Apomorphine Nuciferine PF-6649751 Propylnorapomorphine Rotigotine SKF-89,145 Stepholidine Tavapadon Tetrahydropalmatine PAMs Antagonists
Typical antipsychotics : Butaclamol Chlorpromazine Chlorprothixene Flupentixol (flupenthixol) (+melitracen )Fluphenazine Loxapine Perphenazine (+amitriptyline )Thioridazine Thiothixene Trifluoperazine (+tranylcypromine )Zuclopenthixol Atypical antipsychotics : Asenapine Clorotepine Clotiapine Clozapine DHA-clozapine Fluperlapine Iloperidone Norclozapine Olanzapine (+fluoxetine )Paliperidone Quetiapine Risperidone Zicronapine Ziprasidone Zotepine Others : Ecopipam EEDQ Metitepine (methiothepin) Perlapine SCH-23390
D2 -like
Agonists
Adamantanes : Amantadine Memantine Rimantadine Aminotetralins : 5-OH-DPAT 7-OH-DPAT 8-OH-PBZI Rotigotine UH-232 Ergolines : Bromocriptine Cabergoline Chanoclavine Dihydroergocryptine Epicriptine Ergocornine Lergotrile Lisuride LSD Pergolide Terguride Dihydrexidine derivatives : 2-OH-NPA Ciladopa Dihydrexidine Dinoxyline Phenethylamines : Deoxyepinephrine (N-methyldopamine, epinine) Dopexamine Etilevodopa Ibopamine L -DOPA (levodopa)L -PhenylalanineL -TyrosineMelevodopa Atypical antipsychotics : Alentemol (U-66444B) Aripiprazole (+sertraline )Aripiprazole lauroxil Bifeprunox Brexpiprazole Brilaroxazine Cariprazine F-15063 Lumateperone Norclozapine Others : 3-PPP A-412997 ABT-670 ABT-724 Adrafinil Aplindore Apomorphine Arketamine Armodafinil BP-897 Captodiame CP-226,269 Dizocilpine Esketamine Flibanserin Ketamine Mesulergine Modafinil OSU-6162 Pardoprunox PD-128,907 PD-168,077 PF-219,061 PF-592,379 Phencyclidine Piribedil Pramipexole Preclamol Propylnorapomorphine Pukateine Quinagolide Quinelorane Quinpirole RDS-127 Ro10-5824 Ropinirole Roxindole Salvinorin A SKF-83,959 Sumanirole Talipexole Umespirone WAY-100,635 Antagonists
See also: Receptor/signaling modulators Adrenergics Serotonergics Monoamine reuptake inhibitors Monoamine releasing agents Monoamine metabolism modulators Monoamine neurotoxins