Nabitan ATC code
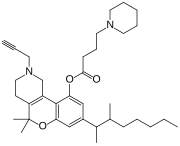
5,5-Dimethyl-8-(3-methyloctan-2-yl)-2-(prop-2-yn-1-yl)-1,3,4,5-tetrahydro-2H -[1]benzopyrano[4,3-c ]pyridin-10-yl 4-(piperidin-1-yl)butanoate
CAS Number PubChem CID ChemSpider UNII Formula C 35 H 52 N 2 O 3 Molar mass −1 3D model (JSmol )
O=C(Oc2cc(cc1OC(C\3=C(/c12)CN(CC/3)CC#C)(C)C)C(C)C(C)CCCCC)CCCN4CCCCC4
InChI=1S/C35H52N2O3/c1-7-9-11-15-26(3)27(4)28-23-31(39-33(38)16-14-21-36-19-12-10-13-20-36)34-29-25-37(18-8-2)22-17-30(29)35(5,6)40-32(34)24-28/h2,23-24,26-27H,7,9-22,25H2,1,3-6H3
Y Key:MCVPMHDADNVRKF-UHFFFAOYSA-N
Y
Nabitan (Nabutam , Benzopyranoperidine , SP-106 , Abbott 40656 ) is a synthetic cannabinoid analog of dronabinol (Marinol).[1] antiemetic and analgesic effects, most likely by binding to and activating the CB1 and CB2 cannabinoid receptors , and reduced intraocular pressure in animal tests, making it potentially useful in the treatment of glaucoma .[2]
Nabitan has the advantage of being water-soluble, unlike most cannabinoid derivatives, and was researched for potential use as an analgesic or sedative ,[3] dronabinol or nabilone were felt to be more useful. However it is sometimes used in research into the potential therapeutic applications of cannabinoids.
References [ ]
^ Razdan RK. The Total Synthesis of Cannabinoids. Wiley-Interscience 1980
^ Razdan RK, Howes JF (1983). "Drugs related to tetrahydrocannabinol". Medicinal Research Reviews . 3 (2): 119–46. doi :10.1002/med.2610030203 . PMID 6134882 . S2CID 31313909 . ^ Archer RA (1974). "The cannabinoids: therapeutic potentials". Annual Reports in Medicinal Chemistry . 9 : 253–9. doi :10.1016/s0065-7743(08)61448-7 . PMID 12307093 .
Cannabinoids
Phytocannabinoids
Cannabichromenes Cannabicyclols Cannabidiols Cannabielsoins Cannabigerols Cannabinols and cannabinodiols Cannabitriols Delta-8-tetrahydrocannabinols Delta-9-tetrahydrocannabinols
Delta-9-THC (THC)
THCH THCP
THCV
Miscellaneous cannabinoids Active metabolites
Endocannabinoids
Arachidonoyl ethanolamide (AEA; anandamide) 2-Arachidonoylglycerol (2-AG) 2-Arachidonyl glyceryl ether (2-AGE; noladin ether) 2-Oleoylglycerol (2-OG) N-Arachidonoyl dopamine (NADA) N-Arachidonylglycine (NAGly) N-Arachidonoyl serotonin (AA-5-HT) Docosatetraenoylethanolamide (DEA) Lysophosphatidylinositol (LPI) Oleamide Oleoylethanolamide (OEA) Palmitoylethanolamide (PEA) RVD-Hpα Stearoylethanolamide (SEA) O-Arachidonoyl ethanolamine (O-AEA; virodhamine) Synthetic
Classical cannabinoids Non-classical Adamantoylindoles Benzimidazoles Benzoylindoles
AM-630 AM-679 AM-694 AM-1241 AM-2233 GW-405,833 (L-768,242) Pravadoline RCS-4 WIN 54,461 Cyclohexylphenols Eicosanoids
ACEA ACPA Methanandamide (AM-356) O-1812 Hydrocarbons Indazole carboxamides Indazole-3- Indole-3-carboxamides Indole-3-carboxylates Naphthoylindazoles Naphthoylindoles Naphthoylpyrroles Naphthylmethylindenes Naphthylmethylindoles Phenylacetylindoles Pyrazolecarboxamides Pyrrolobenzoxazines Quinolinyl esters Tetramethylcyclo- Tetramethylcyclo-
A-796,260 A-834,735 FUB-144 UR-144 XLR-11 XLR-12 Tetramethylcyclo- Others
Allosteric CBR ligands Endocannabinoid (inactivation inhibitors) Anticannabinoids (antagonists/inverse
AM-251 AM-630 AM-6545 BML-190 Drinabant (AVE1625) Hemopressin Ibipinabant (SLV319) JTE-907 LY-320,135 MK-9470 NESS-0327 O-1918 O-2050 Otenabant (CP-945,598) PF-514273 PipISB PSB-SB-487 Rimonabant (SR141716) Rosonabant (E-6776) SR-144,528 Surinabant (SR147778) Taranabant (MK-0364) TM-38837 VCHSR
See also: Cannabinoid receptor modulators (cannabinoids by pharmacology)List of: AM cannabinoids JWH cannabinoids Designer drugs § Synthetic cannabimimetics
Hallucinogens
Psychedelics (5-HT2A
Benzofurans
2C-B-FLY 2CBFly-NBOMe 5-MeO-BFE 5-MeO-DiBF Bromo-DragonFLY F-2 F-22 TFMFly Lyserg‐
1B-LSD 1cP-LSD 1P-ETH-LAD 1P-LSD 1V-LSD 2-Butyllysergamide 3-Pentyllysergamide AL-LAD ALD-52 BU-LAD Diallyllysergamide Dimethyllysergamide ECPLA Ergometrine ETH-LAD IP-LAD LAE-32 LPD-824 LSA LSD LSD-Pip LSH LSM-775 LSZ Methylergometrine MIPLA Methysergide MLD-41 PARGY-LAD PRO-LAD Phenethyl‐
Piperazines Tryptamines
alpha -alkyltryptamines
4,5-DHP-α-MT 5-MeO-α-ET 5-MeO-α-MT α-ET α-MT x -DALT
(Daltacetin) 4-AcO-DALT 5-MeO-DALT DALT x -DET
(Ethacetin) 4-AcO-DET (Ethocin) 4-HO-DET 5-MeO-DET (T-9) DET (Ethocybin) 4-PO-DET x -DiPT
(Ipracetin) 4-AcO-DiPT (Iprocin) 4-HO-DiPT 5-MeO-DiPT DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4-Propionyloxy-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT
(Deprocin) 4-HO-DPT 5-MeO-DPT (The Light) DPT Ibogaine-related
18-MAC 18-MC Coronaridine Ibogaine Ibogamine ME-18-MC Noribogaine Tabernanthine Voacangine x -MET
(Metocin) 4-HO-MET (Metocetin) 4-AcO-MET 5-MeO-MET MET x -MiPT
(Mipracetin) 4-AcO-MiPT (Miprocin) 4-HO-MiPT 5-Me-MiPT (Moxy) 5-MeO-MiPT MiPT Others
4-HO-DBT 4-HO-EPT 4-HO-McPT (Lucigenol) 4-HO-MPMI (Meprocin) 4-HO-MPT 5-MeO-EiPT 5-MeO-MALT 5-MeO-MPMI Aeruginascin Baeocystin DBT EiPT EPT MPT PiPT
Others
AL-38022A ALPHA Dimemebfe Efavirenz Glaucine Lorcaserin M-ALPHA RH-34 Also empathogens in general (e. g.: 5-APB , 5-MAPB , 6-APB and other substituted benzofurans ).
Dissociatives (NMDAR antagonists )
Arylcyclo‐
Ketamine-related
2-Fluorodeschloroketamine Arketamine ((R)-ketamine)Deschloroketamine Ethketamine (N-Ethylnorketamine)Esketamine ((S)-ketamine)Ketamine Methoxetamine Methoxmetamine Methoxyketamine MXiPr Norketamine Tiletamine PCP-related Others
Diarylethylamines Morphinans
Dextrallorphan Dextromethorphan Dextrorphan Racemethorphan Racemorphan Others
2-EMSB 2-MDP 8A-PDHQ Aptiganel Budipine Delucemine Dexoxadrol Dizocilpine Etoxadrol Herkinorin Ibogaine Midafotel NEFA Neramexane Nitrous oxide Noribogaine Perzinfotel RB-64 Remacemide Selfotel Xenon
Deliriants (mAChR antagonists )
Atropine Benactyzine Benzatropine Benzydamine Biperiden Brompheniramine BZ CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,668 Chloropyramine Chlorphenamine Clemastine CS-27349 Cyclizine Cyproheptadine Dicycloverine Dimenhydrinate Diphenhydramine Ditran Doxylamine EA-3167 EA-3443 EA-3580 EA-3834 Elemicin Flavoxate Hyoscyamine JB-318 JB-336 Meclozine Mepyramine Myristicin Orphenadrine Oxybutynin Pheniramine Phenyltoloxamine Procyclidine Promethazine Scopolamine Tolterodine Trihexyphenidyl Tripelennamine Triprolidine WIN-2299 Others
Receptor (ligands )
CB1
Agonists (abridged; see here for more) : 2-AG 2-AGE (noladin ether) 11-Hydroxy-THC α-Amyrin · β-Amyrin AB-CHMINACA AM-1220 AM-1221 AM-1235 AM-2201 AM-2232 Anandamide AZ-11713908 Cannabinol CB-13 CP 47,497 CP 55,940 Dimethylheptylpyran DEA ECG EGCG Epicatechin Gallocatechol (gallocatechin) Honokiol HU-210 JWH-007 JWH-015 JWH-018 JWH-073 Kavain L-759,633 Levonantradol Menabitan Nabilone Nabitan NADA O-1812 Oleamide Pravadoline Serinolamide A THC (dronabinol) UR-144 WIN 55,212-2 Yangonin Antagonists: AM-251 AM-6545 Cannabidiol Cannabigerol Drinabant Falcarinol (carotatoxin) Hemopressin Ibipinabant LY-320,135 MK-9470 NESS-0327 O-2050 Otenabant PF-514273 PipISB Rimonabant Rosonabant Surinabant Taranabant THCV TM-38837 VCHSR Virodhamine CB2
Agonists: 2-AG 2-AGE (noladin ether) 3,3'-Diindolylmethane 4-O-Methylhonokiol α-Amyrin · β-Amyrin A-796,260 A-834,735 A-836,339 AM-1221 AM-1235 AM-1241 AM-2232 Anandamide AZ-11713908 Cannabinol Caryophyllene CB-13 CBS-0550 CP 55,940 GW-405,833 (L-768,242) GW-842,166X HU-308 JTE 7-31 JWH-007 JWH-015 JWH-018 JWH-133 L-759,633 L-759,656 Magnolol MDA-19 Nabitan NADA Olorinab (APD-371) PF-03550096 S-444,823 SER-601 Serinolamide A UR-144 Tedalinab THC (dronabinol) THCV Virodhamine Antagonists: 4-O-Methylhonokiol AM-630 BML-190 Cannabidiol Honokiol JTE-907 SR-144,528 WIN 54,461 WIN 56,098 NAGly GPR18 )
Agonists: Abnormal cannabidiol ACPA AM251 Anandamide Cannabidiol NADGly THC (dronabinol) O-1602 GPR55
Agonists: 2-AGE (noladin ether) Abnormal cannabidiol AM-251 CP 55,940 Lysophosphatidylinositol O-1602 Oleoylethanolamide Palmitoylethanolamide THC (dronabinol) GPR119 Unsorted
Transporter (modulators )
eCBTs
Inhibitors: AM-404 Arachidonoyl serotonin Cannabidiol Guineensine LY-2183240 Paracetamol (acetaminophen) URB-597 VDM-11
Enzyme (modulators )
FAAH MAGL
Inhibitors: IDFP JZL-184 JZL-195 MAFP URB-602 ABHD6 ABHD12
Inhibitors: Betulinic acid Maslinic acid MAFP Oleanolic acid Orlistat (tetrahydrolipstatin) Ursolic acid
Others
Precursors: Phosphatidylethanolamine NAPE Diacylglycerol Others: (directly potentiates activity of 2-AG at CB1 receptor) (FAAH-like anandamide transporter inhibitor)
See also
Receptor/signaling modulators Cannabinoids (cannabinoids by structure)