Piretanide
 | |
| Clinical data | |
|---|---|
| Trade names | Arelix, Eurelix, Tauliz, others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~90%[1] |
| Protein binding | 96% |
| Metabolism | not identified |
| Excretion | Urine (60%), feces (40%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.394 |
| Chemical and physical data | |
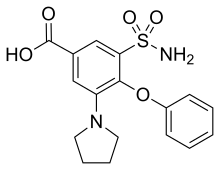
| Formula | C17H18N2O5S |
| Molar mass | 362.40 g·mol−1 |
| 3D model (JSmol) | |
| |
InChI
| |
| | |
Piretanide is a loop diuretic[2] compound by using a then-new method for introducing cyclic amine residues in an aromatic nucleus in the presence of other aromatically bonded functional groups. Studies of piretanide in rats and dogs in comparison with other high-ceiling diuretics such as furosemide and bumetanide found a more suitable dose/response rate (regression line) and a more favourable sodium/potassium excretion ratio. These findings led eventually to studies in man and finally to the introduction as a saluretic and antihypertensive[3] medication in Germany, France, Italy and other countries.
It was made in 1973, patented in 1974, and approved for medical use in 1981.[4]
Brand names[]
Brand names include Arelix, Eurelix, Tauliz.
References[]
- ^ Mutschler, Edited by Rainer F. Greger, H. Knauf, E. Mutschler (1995). Diuretics. Handbook of Experimental Pharmacology (Том 117). Berlin, Heidelberg: Springer Science & Business Media, 2012. p. 517. ISBN 978-3642795657.
{{cite book}}:|first1=has generic name (help) - ^ Musini, Vijaya M.; Rezapour, Pouria; Wright, James M.; Bassett, Ken; Jauca, Ciprian D. (2015-05-22). "Blood pressure-lowering efficacy of loop diuretics for primary hypertension". The Cochrane Database of Systematic Reviews (5): CD003825. doi:10.1002/14651858.CD003825.pub4. ISSN 1469-493X. PMC 7156893. PMID 26000442.
- ^ Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-196-8.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 458. ISBN 9783527607495.
Further reading[]
- Merkel W, Bormann D, Mania D, Muschaweck R, Hropot M (1976). "Piretanide (HOE118): A new high-ceiling sali-diuretic". European Journal of Medicinal Chemistry. 11: 399.
- Merkel W, Mania D, Bormann D (1979). "Selektive Reduktion von Imiden mit funktionellen Gruppen" [Selective reduction of imides in the presence of other function groups]. Liebigs Annalen der Chemie (in German). 1979 (4): 461–9. doi:10.1002/jlac.197919790406.
Categories:
- Diuretics
- Pyrrolidines
- Phenol ethers
- Benzoic acids
- Carbonic anhydrase inhibitors
- NMDA receptor antagonists
- Cardiovascular system drug stubs