Tonazocine ATC code Legal status
In general: non-regulated
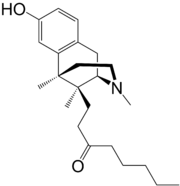
1-[(2S ,6R ,11S )-8-hydroxy-3,6,11-trimethyl-1,2,3,4,5,6-hexahydro-2,6-methano-3-benzazocin-11-yl]octan-3-one
CAS Number PubChem CID ChemSpider UNII Formula C 23 H 35 N O 2 Molar mass −1 3D model (JSmol )
O=C(CCCCC)CC[C@@]3([C@H]2N(CC[C@@]3(c1c(ccc(O)c1)C2)C)C)C
InChI=1S/C23H35NO2/c1-5-6-7-8-18(25)11-12-23(3)21-15-17-9-10-19(26)16-20(17)22(23,2)13-14-24(21)4/h9-10,16,21,26H,5-8,11-15H2,1-4H3/t21-,22+,23+/m0/s1
Key:UDNUCVYCLQJJBY-YTFSRNRJSA-N
Tonazocine (WIN-42,156 ) is an opioid analgesic of the benzomorphan family which made it to phase II clinical trials for the treatment of postoperative pain,[1] partial agonist at both the mu-opioid and delta-opioid receptors , but acting more like an antagonist at the former and more like an agonist at the latter.[2] [3] side effects of other opioids such as adverse effects on the cardiovascular system and respiratory depression , but it can cause sedation (although to a lesser degree of typical opioids), and in some patients it may induce hallucinations (probably via binding to and activating the κ-opioid receptor ).[4]
See also [ ] References [ ]
Analgesics (N02A , N02B )
Opioids
Opiates /opium
Codeine #
Morphine # (+naltrexone )Opium Laudanum Paregoric Semisynthetic
Acetyldihydrocodeine Benzylmorphine Buprenorphine (+naloxone )Butorphanol Desomorphine Diamorphine (heroin) Dihydrocodeine (+paracetamol )Dihydromorphine Etorphine Ethylmorphine Hydrocodone (+paracetamol , +ibuprofen , +aspirin )Hydromorphinol Hydromorphone Levorphanol Metopon Nalbuphine Nicocodeine Nicodicodine Nicomorphine Oxycodone (+paracetamol , +aspirin , +ibuprofen , +naloxone , )Oxymorphone Papaveretum Thebacon Synthetic
Paracetamol -type
Acetanilide ‡ Bucetin ‡ ‡ Paracetamol (acetaminophen) #
+aspirin/caffeine +codeine +hydrocodone +metoclopramide +oxycodone +propyphenazone/caffeine ‡ Phenacetin ‡ Propacetamol ‡ NSAIDs
Propionates
Benoxaprofen ‡ Fenoprofen Flurbiprofen Ibuprofen # (Dexibuprofen )Ketoprofen (Dexketoprofen )Loxoprofen Naproxen Oxaprozin Suprofen Tiaprofenic acid Zaltoprofen Oxicams
Isoxicam Lornoxicam Meloxicam Piroxicam Tenoxicam Acetates
Acemetacin Bromfenac Diclofenac Etodolac Indometacin (Indometacin farnesil )Ketorolac Sulindac Tolmetin Zomepirac ‡ COX-2 inhibitors Fenamates
Flufenamic acid Meclofenamic acid Mefenamic acid Tolfenamic acid Salicylates
Aspirin (acetylsalicylic acid) # (+paracetamol/caffeine )Aloxiprin Benorylate Carbasalate calcium Choline salicylate Diflunisal Dipyrocetyl Ethenzamide Guacetisal Imidazole salicylate Magnesium salicylate Morpholine salicylate Potassium salicylate Salicin Salicylamide Salsalate Sodium salicylate Wintergreen (methyl salicylate ) Pyrazolones
Aminophenazone ‡ Ampyrone Metamizole (dipyrone) Nifenazone Phenazone Propyphenazone (+paracetamol/caffeine ) Others
Benzydamine Floctafenine Glafenine Nabumetone Nimesulide Proquazone
Cannabinoids
Cannabidiol Cannabis Nabilone Nabiximols Tetrahydrocannabinol (dronabinol) Ion channel
Calcium blockers
Alcohol (ethanol) Gabapentin Gabapentin enacarbil Leconotide Mirogabalin Pregabalin Ziconotide Sodium blockers
Carbamazepine Lacosamide Local anesthetics (e.g., cocaine , lidocaine )Mexiletine Nefopam Tricyclic antidepressants (e.g., amitriptyline # )Nav 1.7/1.8-selective: DSP-2230 § Funapide § PF-05089771 § Potassium openers
Myorelaxants
Carisoprodol Chlorzoxazone Cyclobenzaprine Mephenoxalone Methocarbamol Orphenadrine Others
# WHO-EM ‡ Withdrawn from marketClinical trials :
† Phase III § Never to phase III
Hallucinogens
Psychedelics (5-HT2A
Benzofurans
2C-B-FLY 2CBFly-NBOMe 5-MeO-BFE 5-MeO-DiBF Bromo-DragonFLY F-2 F-22 TFMFly Lyserg‐
1B-LSD 1cP-LSD 1P-ETH-LAD 1P-LSD 1V-LSD 2-Butyllysergamide 3-Pentyllysergamide AL-LAD ALD-52 BU-LAD Diallyllysergamide Dimethyllysergamide ECPLA Ergometrine ETH-LAD IP-LAD LAE-32 LPD-824 LSA LSD LSD-Pip LSH LSM-775 LSZ Methylergometrine MIPLA Methysergide MLD-41 PARGY-LAD PRO-LAD Phenethyl‐
Piperazines Tryptamines
alpha -alkyltryptamines
4,5-DHP-α-MT 5-MeO-α-ET 5-MeO-α-MT α-ET α-MT x -DALT
(Daltacetin) 4-AcO-DALT 5-MeO-DALT DALT x -DET
(Ethacetin) 4-AcO-DET (Ethocin) 4-HO-DET 5-MeO-DET (T-9) DET (Ethocybin) 4-PO-DET x -DiPT
(Ipracetin) 4-AcO-DiPT (Iprocin) 4-HO-DiPT 5-MeO-DiPT DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4-Propionyloxy-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT
(Deprocin) 4-HO-DPT 5-MeO-DPT (The Light) DPT Ibogaine-related
18-MAC 18-MC Coronaridine Ibogaine Ibogamine ME-18-MC Noribogaine Tabernanthine Voacangine x -MET
(Metocin) 4-HO-MET (Metocetin) 4-AcO-MET 5-MeO-MET MET x -MiPT
(Mipracetin) 4-AcO-MiPT (Miprocin) 4-HO-MiPT 5-Me-MiPT (Moxy) 5-MeO-MiPT MiPT Others
4-HO-DBT 4-HO-EPT 4-HO-McPT (Lucigenol) 4-HO-MPMI (Meprocin) 4-HO-MPT 5-MeO-EiPT 5-MeO-MALT 5-MeO-MPMI Aeruginascin Baeocystin DBT EiPT EPT MPT PiPT
Others
AL-38022A ALPHA Dimemebfe Efavirenz Glaucine Lorcaserin M-ALPHA RH-34 Also empathogens in general (e. g.: 5-APB , 5-MAPB , 6-APB and other substituted benzofurans ).
Dissociatives (NMDAR antagonists )
Arylcyclo‐
Ketamine-related PCP-related Others
Diarylethylamines Morphinans
Dextrallorphan Dextromethorphan Dextrorphan Racemethorphan Racemorphan Others
Deliriants (mAChR antagonists )
Atropine Benactyzine Benzatropine Benzydamine Biperiden Brompheniramine BZ CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,668 Chloropyramine Chlorphenamine Clemastine CS-27349 Cyclizine Cyproheptadine Dicycloverine Dimenhydrinate Diphenhydramine Ditran Doxylamine EA-3167 EA-3443 EA-3580 EA-3834 Elemicin Flavoxate Hyoscyamine JB-318 JB-336 Meclozine Mepyramine Myristicin Orphenadrine Oxybutynin Pheniramine Phenyltoloxamine Procyclidine Promethazine Scopolamine Tolterodine Trihexyphenidyl Tripelennamine Triprolidine WIN-2299 Others
Cannabinoids (CB1 agonists)
Natural
Salvinorin A THC (Dronabinol) THCV Synthetic
D2 agonists
Apomorphine Aporphine Bromocriptine Cabergoline Lisuride LSD Memantine Nuciferine Pergolide Phenethylamine Piribedil Pramipexole Ropinirole Rotigotine Salvinorin A Also indirect D2 agonists, such as dopamine reuptake inhibitors (cocaine , methylphenidate ), releasing agents (amphetamine , methamphetamine ), and precursors (levodopa ). GABAA
CI-966 Eszopiclone Ibotenic acid Muscimol (Amanita muscaria Zaleplon Zolpidem Zopiclone Inhalants (Mixed MOA )
Aliphatic hydrocarbons
Butane Gasoline Kerosene Propane Aromatic hydrocarbons
Ethers
Haloalkanes
Chlorofluorocarbons Chloroform κOR agonists
2-EMSB Alazocine Bremazocine Butorphan Butorphanol Cyclazocine Cyclorphan Cyprenorphine Diprenorphine Enadoline Herkinorin Heroin HZ-2 Ibogaine Ketazocine Levallorphan Levomethorphan Levorphanol LPK-26 Metazocine Morphine Nalbuphine Nalmefene Nalorphine Noribogaine Oxilorphan Pentazocine Phenazocine Proxorphan Racemethorphan Racemorphan Salvinorin A Spiradoline Tifluadom U-50488 U-69,593 Xorphanol Oneirogens
Calea zacatechichi Silene capensis Galantamine Others
Glaucine Isoaminile Noscapine Pukateine
MOR DOR KOR NOP
Agonists: Buprenorphine Cebranopadol Dihydroetorphine Etorphine Levomethorphan Levorphanol MCOPPB NNC 63-0532 Nociceptin (orphanin FQ) Norbuprenorphine Racemethorphan Racemorphan Ro64-6198 SR-16435 Sunobinop (S-117957) Unsorted
β-Casomorphins Amidorphin Cytochrophin-4 Gliadorphin (gluteomorphin) Gluten exorphins Hemorphins Kava constituentsNEM Neoendorphins Nepetalactone (catnip )Rubiscolins Others
Enkephalinase inhibitors :Amastatin Candoxatril D -PhenylalanineEcadotril (sinorphan) Kelatorphan Racecadotril (acetorphan) RB-101 RB-120 RB-3007 Selank Semax Spinorphin Thiorphan Tynorphin Ubenimex (bestatin) Propeptides: β-Lipotropin (proendorphin) Prodynorphin Proenkephalin Proopiomelanocortin (POMC) Others: Kyotorphin (met-enkephalin releaser/degradation stabilizer)