Acyl chloride
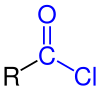
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
Nomenclature[]
Where the acyl chloride moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting -yl chloride for -ic acid. Thus:
- acetyl chloride CH3COCl
- benzoyl chloride C6H5COCl
When other functional groups take priority, acyl chlorides are considered prefixes — chlorocarbonyl-:[1]
- (chlorocarbonyl)acetic acid ClOCCH2COOH
Properties[]
Lacking the ability to form hydrogen bonds, acyl chlorides have lower boiling and melting points than similar carboxylic acids. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most carbonyl compounds, infrared spectroscopy reveals a band near 1750 cm−1.
The simplest stable acyl chloride is acetyl chloride; formyl chloride is not stable at room temperature, although it can be prepared at –60 °C or below.[2][3] Acyl chloride hydrolyzes (reacts with water).

Synthesis[]
Industrial routes[]
The industrial route to acetyl chloride involves the reaction of acetic anhydride with hydrogen chloride:[5]
- (CH3CO)2O + HCl → CH3COCl + CH3CO2H
Propionyl chloride is produced by chlorination of propionic acid with phosgene:[6]
- CH3CH2CO2H + COCl2 → CH3CH2COCl + HCl + CO2
Benzoyl chloride is produced by the partial hydrolysis of benzotrichloride:[7]
- C6H5CCl3 + H2O → C6H5C(O)Cl + 2 HCl
Laboratory methods: thionyl chloride[]
In the laboratory, acyl chlorides are generally prepared by treating carboxylic acids with thionyl chloride (SOCl2).[8] The reaction is catalyzed by dimethylformamide and other additives.[9]

Thionyl chloride[10] is a well-suited reagent as the by-products (HCl, SO2) are gases and residual thionyl chloride can be easily removed as a result of its low boiling point (76 °C). The reaction with thionyl chloride is catalyzed by dimethylformamide.[11]
Laboratory methods: phosphorus chlorides[]
Phosphorus trichloride (PCl3) is also popular,[12] phosphorus pentachloride (PCl5).[13][14] although excess reagent is required.[9] Phosphorus pentachloride is also effective but only one chloride is transferred:
- RCO2H + PCl5 → RCOCl + POCl3 + HCl
Laboratory methods: oxalyl chloride[]
Another method involves the use of oxalyl chloride:
- RCO2H + ClCOCOCl → RCOCl + CO + CO2 + HCl
The reaction is catalysed by dimethylformamide (DMF), which reacts with oxalyl chloride in the first step to give an iminium intermediate, which reacts with the carboxylic acid, abstracting an oxide, and regenerating the DMF catalyst.[11] Relative to thionyl chloride, oxalyl chloride is more expensive but also a milder reagent and therefore more selective.
Other laboratory methods[]
Acid chlorides can be used as a chloride source.[15] Thus acetyl chloride can be distilled from a mixture of benzoyl chloride and acetic acid:[9]
- CH3CO2H + C6H5COCl → CH3COCl + C6H5CO2H
Other methods that do not form HCl include the Appel reaction:[16]
- RCO2H + Ph3P + CCl4 → RCOCl + Ph3PO + HCCl3
Another is the use of cyanuric chloride:[17]
- RCO2H + C3N3Cl3 → RCOCl + C3N3Cl2OH
Reactions[]
Acyl chloride are reactive, versatile reagents.[18] Acyl chlorides have a greater reactivity than other carboxylic acid derivatives like acid anhydrides, esters or amides:
Nucleophilic reactions[]
Acid chlorides are useful for the preparation of amides, esters, anhydrides. These reactions generate chloride, which can be undesirable. Acyl chlorides hydrolyze, yielding the carboxylic acid:
This hydrolysis is usually a nuisance rather than intentional. Acyl chlorides are used to prepare acid anhydrides, amides and esters, by reacting acid chlorides with: a salt of a carboxylic acid, an amine, or an alcohol, respectively.
Mechanism[]
The alcoholysis of acyl halides (the alkoxy-dehalogenation) is believed to proceed via an SN2 mechanism (Scheme 10).[19] However, the mechanism can also be tetrahedral or SN1 in highly polar solvents[20] (while the SN2 reaction involves a concerted reaction, the tetrahedral addition-elimination pathway involves a discernible intermediate).[21]

Base, e.g. pyridine or N,N-dimethylformamide, catalyze acylations.[14][11] These reagents activate the acyl chloride via an nucleophilic catalysis mechanism. The amine attacks the carbonyl bond and presumably[22] forms first a transient tetrahedral intermediate and afterwards, by the displacement of the leaving group, a quaternary acylammonium salt. This quaternary acylammonium salt is more susceptible to attack by alcohols or other nucleophiles.
The use of two phases (aqueous for amine, organic for acyl chloride) is called Schotten-Baumann reaction. This approach is used in the preparation of nylon via the so-called nylon rope trick.[23]).
Conversion to ketones[]
Carbon nucleophiles such as Grignard reagents, convert acyl chlorides to ketones, which in turn are susceptible to the attack by second equivalent to yield the tertiary alcohol. The reaction of acyl halides with certain organocadmium reagents stops at the ketone stage.[24] The reaction with Gilman reagents also afford ketones, reflecting the low nucleophilicity of these lithium diorganocopper compounds.[14]
Reduction[]
Acyl chlorides are reduced by lithium aluminium hydride and diisobutylaluminium hydride to give primary alcohols. Lithium tri-tert-butoxyaluminium hydride, a bulky hydride donor, reduces acyl chlorides to aldehydes, as does the Rosenmund reduction using hydrogen gas over a poisoned palladium catalyst.[25]

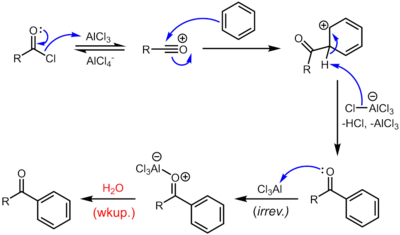
Acylation of arenes[]
With Lewis acid catalysts like ferric chloride or aluminium chloride, acyl chlorides participate in Friedel-Crafts acylations, to give aryl ketones:[12][14]
Because of the harsh conditions and the reactivity of the intermediates, this otherwise quite useful reaction tends to be messy, as well as environmentally unfriendly.
Oxidative addition[]
Acyl chlorides react with low-valent metal centers. Illustrative is the oxidative addition of acetyl chloride to Vaska's complex, converting square planar Ir(I) to octahedral Ir(III):[26]
- IrCl(CO)(PPh3)2 + CH3COCl → CH3COIrCl2(CO)(PPh3)2
Hazards[]
Low molecular weight acyl chlorides are often lachrymators, and they react violently with water, alcohols, and amines.
References[]
- ^ Nomenclature of Organic Chemistry, R-5.7.6 Acid halides
- ^ Sih, John C. (2001-04-15), "Formyl Chloride", in John Wiley & Sons, Ltd (ed.), Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, doi:10.1002/047084289x.rf026, ISBN 9780471936237
- ^ Richard O.C. Norman; James M. Coxon (16 September 1993). Principles of Organic Synthesis, 3rd Edition. CRC Press. p. 371. ISBN 978-0-7487-6162-3.
- ^ Wang, Hong-Yong; Xie, Min-Hao; Luo, Shi-Neng; Zou, Pei; Liu, Ya-Ling (2009). "3,5-Dinitrobenzoyl chloride". Acta Crystallographica Section e Structure Reports Online. 65 (10): o2460. doi:10.1107/S1600536809036228. PMC 2970283. PMID 21577915.
- ^ US patent 5672749, Phillip R. DeVrou, W. Bryan Waites, Robert E. Young, "Process for preparing acetyl chloride"
- ^ Samel, Ulf-Rainer; Kohler, Walter; Gamer, Armin Otto; Keuser, Ullrich (2005). "Propionic acid and derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_223.
- ^ Maki, Takao; Takeda, Kazuo (2002). "Benzoic acid and derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555.
- ^ Helferich, B.; Schaefer, W. (1929). "n-Butyrl chloride". Organic Syntheses. 9: 32. doi:10.15227/orgsyn.009.0032.
- ^ Jump up to: a b c Martin Ansell (1972). "Preparation of acyl halides". In Saul Patai (ed.). Acyl Halides. PATAI'S Chemistry of Functional Groups. doi:10.1002/9780470771273.ch2.
- ^ J. S. Pizey, Synthetic Reagents, Vol. 1, Halsted Press, New York, 1974.
- ^ Jump up to: a b c Clayden, Jonathan (2001). Organic chemistry. Oxford: Oxford University Press. pp. 276–296. ISBN 0-19-850346-6.
- ^ Jump up to: a b Allen, C. F. H.; Barker, W. E. (1932). "Desoxybenzoin". Organic Syntheses. 12: 16. doi:10.15227/orgsyn.012.0016.
- ^ Adams, Roger (1923). "p-Nitrobenzoyl Chloride". Organic Syntheses. 3: 75. doi:10.15227/orgsyn.003.0075.
- ^ Jump up to: a b c d Boyd, Robert W.; Morrison, Robert (1992). Organic Chemistry. Englewood Cliffs, N.J: Prentice Hall. pp. 666–762. ISBN 0-13-643669-2.
- ^ L. P. Kyrides (1940). "Fumaryl Chloride". Organic Syntheses. 20: 51. doi:10.15227/orgsyn.020.0051.
- ^ "Triphenylphosphine-carbon tetrachloride Taschner, Michael J. e-EROS: Encyclopedia of Reagents for Organic Synthesis, 2001
- ^ K. Venkataraman; D. R. Wagle (1979). "Cyanuric chloride : a useful reagent for converting carboxylic acids into chlorides, esters, amides and peptides". Tetrahedron Lett. 20 (32): 3037–3040. doi:10.1016/S0040-4039(00)71006-9.
- ^ Sonntag, Norman O. V. (1953-04-01). "The Reactions of Aliphatic Acid Chlorides". Chemical Reviews. 52 (2): 237–416. doi:10.1021/cr60162a001. ISSN 0009-2665.
- ^ Bentley, T. William; Llewellyn, Gareth; McAlister, J. Anthony (January 1996). "SN2 Mechanism for Alcoholysis, Aminolysis, and Hydrolysis of Acetyl Chloride". The Journal of Organic Chemistry. 61 (22): 7927–7932. doi:10.1021/jo9609844. ISSN 0022-3263. PMID 11667754.
- ^ C. H. Bamford and C. F. H. Tipper, Comprehensive Chemical Kinetics: Ester Formation and Hydrolysis and Related Reactions, Elsevier, Amsterdam, 1972.
- ^ Fox, Joseph M.; Dmitrenko, Olga; Liao, Lian-an; Bach, Robert D. (October 2004). "Computational Studies of Nucleophilic Substitution at Carbonyl Carbon: the S N 2 Mechanism versus the Tetrahedral Intermediate in Organic Synthesis". The Journal of Organic Chemistry. 69 (21): 7317–7328. doi:10.1021/jo049494z. ISSN 0022-3263. PMID 15471486.
- ^ Hubbard, Patricia; Brittain, William J. (February 1998). "Mechanism of Amine-Catalyzed Ester Formation from an Acid Chloride and Alcohol". The Journal of Organic Chemistry. 63 (3): 677–683. doi:10.1021/jo9716643. ISSN 0022-3263. PMID 11672060.
- ^ Morgan, Paul W.; Kwolek, Stephanie L. (April 1959). "The nylon rope trick: Demonstration of condensation polymerization". Journal of Chemical Education. 36 (4): 182. Bibcode:1959JChEd..36..182M. doi:10.1021/ed036p182. ISSN 0021-9584.
- ^ David A. Shirley (2011). "The Synthesis of Ketones from Acid Halides and Organometallic Compounds of Magnesium, Zinc, and Cadmium". Org. Reactions. doi:10.1002/0471264180.or008.02.
- ^ William Reusch. "Carboxylic Acid Derivatives". VirtualText of Organic Chemistry. Michigan State University. Archived from the original on 2016-05-16. Retrieved 2009-02-19.
- ^ Hartwig, John (2010). Organotransition Metal Chemistry: From Bonding to Catalysis. New York: University Science Books. p. 1160. ISBN 978-1-938787-15-7.
- Acyl chlorides
- Functional groups