Allura Red AC
| |
| Names | |
|---|---|
| Preferred IUPAC name
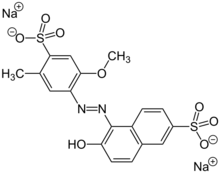
Disodium 6-hydroxy-5-[(2-methoxy-5-methyl-4-sulfonatophenyl)diazenyl]naphthalene-2-sulfonate | |
| Other names
Disodium 6-hydroxy-5-[(2-methoxy-5-methyl-4-sulfophenyl)azo]-2-naphthalenesulfonate
Allura Red Food Red 17 C.I. 16035 FD&C Red 40 E129 2-Naphthalenesulfonic acid, 6-hydroxy-5-((2-methoxy-5-methyl-4-sulfophenyl)azo)-, disodium salt | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.043.047 |
| E number | E129 (colours) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H14N2Na2O8S2 | |
| Molar mass | 496.42 g·mol−1 |
| Appearance | Red powder |
| Melting point | > 300 °C (572 °F; 573 K) |
| Hazards | |
| NFPA 704 (fire diamond) | 
1
1
0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Allura Red AC is a red azo dye that goes by several names, including FD&C Red 40.[1] It is used as a food dye and has the E number E129.
It is usually supplied as its red sodium salt, but can also be used as the calcium and potassium salts. These salts are soluble in water. In solution, its maximum absorbance lies at about 504 nm.[2]: 921
Byproducts of the petroleum industry are used to produce Allura Red, FD&C Red No. 40 is manufactured by coupling diazotized 5-amino-4-methoxy-2-toluenesulfonic acid with 6-hydroxy-2-naphthalene sulfonic acid.[EPA/Office of Pollution Prevention and Toxics; High Production Volume (HPV) Challenge Program's Robust Summaries and Test Plans. 2-Naphthalenesulfonic acid, 6-hydroxy-5-.[3]
Use as a consumable coloring agent[]
Allura Red AC is a popular dye used worldwide. Annual production in 1980 was greater than 2.3 million kilograms.[4]
The European Union approves Allura Red AC as a food colorant, but EU countries' local laws banning food colorants are preserved.[5] In the United States, Allura Red AC is approved by the FDA for use in cosmetics, drugs, and food. When prepared as a lake it is disclosed as Red 40 Lake or Red 40 Aluminum Lake. It is used in some tattoo inks and is used in many products, such as cotton candy, soft drinks, cherry flavored products, children's medications, and dairy products. It is occasionally used to dye medicinal pills, such as the antihistamine fexofenadine, for purely aesthetic reasons.[6] It is by far the most commonly used red dye in the United States, completely replacing amaranth (Red 2) and also replacing erythrosine (Red 3) in most applications due to the negative health effects of those two dyes.
Studies on safety[]


Allura Red has been heavily studied by food safety groups in North America and Europe, and remains in wide use.
The UK's Food Standards Agency commissioned a study of six food dyes (tartrazine, Allura red, Ponceau 4R, Quinoline Yellow, sunset yellow, carmoisine (dubbed the "Southampton 6")), and sodium benzoate (a preservative) on children in the general population, who consumed them in beverages.[7][8] The study found "a possible link between the consumption of these artificial colours and a sodium benzoate preservative and increased hyperactivity" in the children;[7][8] the advisory committee to the FSA that evaluated the study also determined that because of study limitations, the results could not be extrapolated to the general population, and further testing was recommended.[7]
The European Food Safety Authority, with a stronger emphasis on the precautionary principle, required labelling and temporarily reduced the acceptable daily intake (ADI) for the food colorings; the UK FSA called for voluntary withdrawal of the colorings by food manufacturers.[7][8] However, in 2009, the EFSA re-evaluated the data at hand and determined that "the available scientific evidence does not substantiate a link between the color additives and behavioral effects",[7] and in 2014, after further review of the data, the European Food Safety Authority (EFSA) restored the prior ADI levels.[9] In 2015, the EFSA found that the exposure estimates did not exceed the ADI of 7 mg/kg per day in any population.[10]
The US FDA did not make changes following the publication of the Southampton study, but following a citizen petition filed by the Center for Science in the Public Interest in 2008, requesting the FDA ban several food additives, the FDA commenced a review of the available evidence, and still made no changes.[7]
Allura Red AC was at one time banned in Denmark, Belgium, France, and Switzerland, and was also banned in Sweden until the country joined the European Union in 1994.[11][failed verification] In Norway and Iceland, it was banned between 1978 and 2001, a period in which azo dyes were only legally used in alcoholic beverages and some fish products.[12]
References[]
- ^ "From Shampoo to Cereal: Seeing to the Safety of Color Additives". Archived from the original on 2008-01-15. Retrieved 2008-06-04.CS1 maint: bot: original URL status unknown (link) "Food Color Facts". Archived from the original on 2007-10-01. Retrieved 2006-08-18.CS1 maint: bot: original URL status unknown (link)
- ^ Zvi Rappoport, ed. (2004). The Chemistry of Phenols. Chichester: John Wiley & Sons. ISBN 9780470869451.
- ^ PubChem. "Allura Red AC". pubchem.ncbi.nlm.nih.gov. Retrieved 2021-10-21.
- ^ Sharma, Vinita; McKone, Harold T.; Markow, Peter G. (2011). "A Global Perspective on the History, Use, and Identification of Synthetic Food Dyes". Journal of Chemical Education. 88: 24–28. doi:10.1021/ed100545v.
- ^ European Parliament and Council Directive 94/36/EC of 30 June 1994 on colours for use in foodstuffs
- ^ "FD&C Red No. 40 (Inactive Ingredient)".
- ^ a b c d e f FDA (March 30, 2011). "Background Document for the Food Advisory Committee: Certified Color Additives in Food and Possible Association with Attention Deficit Hyperactivity Disorder in Children:date" (PDF). Archived from the original (PDF) on 2017-05-02. Cite journal requires
|journal=(help) - ^ a b c Sarah Chapman of Chapman Technologies on behalf of Food Standards Agency in Scotland. March 2011 [Guidelines on approaches to the replacement of Tartrazine, Allura Red, Ponceau 4R, Quinoline Yellow, Sunset Yellow and Carmoisine in food and beverages]
- ^ EFSA Panel on Food Additives and Nutrient Sources added to food (ANS) Reconsideration of the temporary ADI and refined exposure assessment for Sunset Yellow FCF (E 110) EFSA Journal 2014;12(7):3765 . doi:10.2903/j.efsa.2014.3765
- ^ "Refined exposure assessment for Allura Red AC (E 129) | European Food". Efsa.europa.eu. Retrieved 2018-09-08.
- ^ "E129", UK Food Guide, a British food additives website. Last retrieved 20 May 2007.
- ^ "Norwegian Food Safety Authority". Archived from the original on 2012-08-04. Retrieved 2007-07-09.
External links[]
- 2-Naphthols
- Azo dyes
- Benzenesulfonates
- Food colorings
- Naphthalenesulfonates
- Organic sodium salts
- E-number additives