Antiarrhythmic agent
Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a group of pharmaceuticals that are used to suppress abnormal rhythms of the heart (cardiac arrhythmias), such as atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular fibrillation.
Many attempts have been made to classify antiarrhythmic agents. The problem arises from the fact that many of the antiarrhythmic agents have multiple modes of action, making any classification imprecise.
Vaughan Williams classification[]
The Vaughan Williams classification[1] was introduced in 1970 by Miles Vaughan Williams.[2]
Vaughan Williams was a pharmacology tutor at Hertford College, Oxford. One of his students, Bramah N. Singh,[3] contributed to the development of the classification system. The system is therefore sometimes known as the Singh-Vaughan Williams classification.
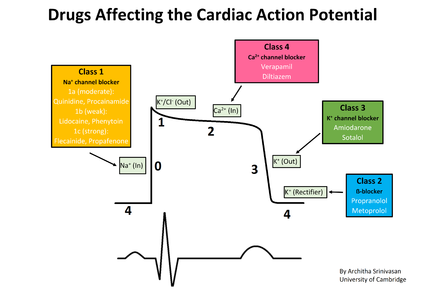
The five main classes in the Vaughan Williams classification of antiarrhythmic agents are:
- Class I agents interfere with the sodium (Na+) channel.
- Class II agents are anti-sympathetic nervous system agents. Most agents in this class are beta blockers.
- Class III agents affect potassium (K+) efflux.
- Class IV agents affect calcium channels and the AV node.
- Class V agents work by other or unknown mechanisms.
With regard to management of atrial fibrillation, classes I and III are used in rhythm control as medical cardioversion agents, while classes II and IV are used as rate-control agents.
| Class | Known as | Examples | Mechanism | Medical uses[4] |
|---|---|---|---|---|
| Ia | Fast-channel blockers | Na+ channel block (intermediate association/dissociation) and K+ channel blocking effect.
Class 1a prolong the action potential and has intermediate effect on the 0 phase of depolarization |
| |
| Ib | Na+ channel block (fast association/dissociation).
Class 1b shorten the action potential of myocardial cell and has weak effect on initiation of phase 0 of depolarization |
| ||
| Ic |
|
Na+ channel block (slow association/dissociation).
Class 1c do not affect action potential duration and have the strongest effect on the initiation phase 0 of depolarization |
| |
| II | Beta-blockers | Beta blocker Propranolol also has some sodium channel-blocking effects. |
| |
| III | Potassium Channel Blockers | K+ channel blocker
Sotalol is also a beta blocker[5] Amiodarone has Class III mostly, but also I, II, & IV activity[6] |
| |
| IV | Calcium Channel Blockers | Ca2+ channel blocker |
| |
| V | Work by other or unknown mechanisms (direct nodal inhibition) |
|
Class I agents[]
The class I antiarrhythmic agents interfere with the sodium channel. Class I agents are grouped by what effect they have on the Na+ channel, and what effect they have on cardiac action potentials.
Class I agents are called membrane-stabilizing agents, "stabilizing" referring to the decrease of excitogenicity of the plasma membrane which is brought about by these agents. (Also noteworthy is that a few class II agents like propranolol also have a membrane stabilizing effect.)
Class I agents are divided into three groups (Ia, Ib, and Ic) based upon their effect on the length of the action potential.[9][10]
- Ia lengthens the action potential (right shift)
- Ib shortens the action potential (left shift)
- Ic does not significantly affect the action potential (no shift)
Class Ia

Class Ib

Class Ic
Class II agents[]
Class II agents are conventional beta blockers. They act by blocking the effects of catecholamines at the β1-adrenergic receptors, thereby decreasing sympathetic activity on the heart, which reduces intracellular cAMP levels and hence reduces Ca2+ influx. These agents are particularly useful in the treatment of supraventricular tachycardias. They decrease conduction through the AV node.
Class II agents include atenolol, esmolol, propranolol, and metoprolol.
Class III agents[]

Class III agents predominantly block the potassium channels, thereby prolonging repolarization.[11] Since these agents do not affect the sodium channel, conduction velocity is not decreased. The prolongation of the action potential duration and refractory period, combined with the maintenance of normal conduction velocity, prevent re-entrant arrhythmias. (The re-entrant rhythm is less likely to interact with tissue that has become refractory). The class III agents exhibit reverse-use dependence (their potency increases with slower heart rates, and therefore improves maintenance of sinus rhythm). Inhibiting potassium channels, slowing repolarization, results in slowed atrial-ventricular myocyte repolarization. Class III agents have the potential to prolong the QT interval of the EKG, and may be proarrhythmic (more associated with development of polymorphic VT).
Class III agents include: bretylium, amiodarone, ibutilide, sotalol, dofetilide, vernakalant and dronedarone.
Class IV agents[]
Class IV agents are slow non-dihydropyridine calcium channel blockers. They decrease conduction through the AV node, and shorten phase two (the plateau) of the cardiac action potential. They thus reduce the contractility of the heart, so may be inappropriate in heart failure. However, in contrast to beta blockers, they allow the body to retain adrenergic control of heart rate and contractility.
Class IV agents include verapamil and diltiazem.
Class V and others[]
Since the development of the original Vaughan Williams classification system, additional agents have been used that do not fit cleanly into categories I through IV. Such agents include:
- Digoxin, which decreases conduction of electrical impulses through the AV node and increases vagal activity via its central action on the central nervous system, via indirect action, leads to an increase in acetylcholine production, stimulating M2 receptors on AV node leading to an overall decrease in speed of conduction.
- Adenosine is used intravenously for terminating supraventricular tachycardias.[12]
- Magnesium sulfate, an antiarrhythmic drug, but only against very specific arrhythmias [13] which has been used for torsades de pointes.[14][15]
- Trimagnesium dicitrate (anhydrous) as powder or powder caps in pure condition, better bioavailability than ordinary MgO[16]
History[]
The initial classification system had 4 classes, although their definitions different from the modern classification. Those proposed in 1970 were:[2]
- Drugs with a direct membrane action: the prototype was quinidine, and lignocaine was a key example. Differing from other authors, Vaughan-Williams describe the main action as a slowing of the rising phase of the action potential.
- Sympatholytic drugs (drugs blocking the effects of the sympathetic nervous system): examples included bretylium and adrenergic beta-receptors blocking drugs. This is similar to the modern classification, which focuses on the latter category.
- Compounds that prolong the action potential: matching the modern classification, with the key drug example being amiodarone, and a surgical example being thyroidectomy. This was not a defining characteristic in an earlier review by Charlier et al. (1968),[17] but was supported by experimental data presented by Vaughan Williams (1970).[2]: 461 The figure illustrating these findings was also published in the same year by Singh and Vaughan Williams.[18]
- Drugs acting like dephenylhydantoin (DPH): mechanism of action unknown, but others had attributed its cardiac action to an indirect action on the brain;[19] this drug is better known as antiepileptic drug phenytoin.
Sicilian gambit classification[]
Another approach, known as the "Sicilian gambit", placed a greater approach on the underlying mechanism.[20][21][22]
It presents the drugs on two axes, instead of one, and is presented in tabular form. On the Y axis, each drug is listed, in roughly the Singh-Vaughan Williams order. On the X axis, the channels, receptors, pumps, and clinical effects are listed for each drug, with the results listed in a grid. It is, therefore, not a true classification in that it does not aggregate drugs into categories.[23]
A modernized Oxford classification by Lei, Huang, Wu and Terrar[]

A recent publication has now emerged with a fully modernised drug classification.[24] This preserves the simplicity of the original Vaughan Williams framework while capturing subsequent discoveries of sarcolemmal, sarcoplasmic reticular and cytosolic biomolecules. The result is an expanded but pragmatic classification that encompasses approved and potential anti-arrhythmic drugs. This will aid our understanding and clinical management of cardiac arrhythmias and facilitate future therapeutic developments. It starts by considering the range of pharmacological targets, and tracks these to their particular cellular electrophysiological effects. It retains but expands the original Vaughan Williams classes I to IV, respectively covering actions on Na+ current components, autonomic signalling, K+ channel subspecies, and molecular targets related to Ca2+ homeostasis. It now introduces new classes incorporating additional targets, including:
- Class 0: ion channels involved in automaticity
- Class V: mechanically sensitive ion channels
- Class VI: connexins controlling electrotonic cell coupling
- Class VII: molecules underlying longer term signalling processes affecting structural remodeling.
It also allows for multiple drug targets/actions and adverse pro-arrhythmic effects. The new scheme will additionally aid development of novel drugs under development and is illustrated here.
See also[]
- Cardiac Arrhythmia Suppression Trial (CAST)
- Electrocardiogram
- Proarrhythmic agent
References[]
- ^ Rang, Humphrey P.; Ritter, James M.; Flower, Rod J.; Henderson, Graeme (2012). Rang and Dale's pharmacology (7th ed.). Elsevier. p. 255. ISBN 9780702034718.
- ^ Jump up to: a b c Vaughan Williams, EM (1970) Classification of antiarrhythmic drugs. In Symposium on Cardiac Arrhythmias (Eds. Sandoe E, Flensted- Jensen E, Olsen KH). Astra, Elsinore. Denmark (1970)
- ^ Kloner RA (2009). "A Salute to Our Founding Editor-in-Chief Bramah N. Singh, MD, DPhil, DSc, FRCP". Journal of Cardiovascular Pharmacology and Therapeutics. 14 (3): 154–56. doi:10.1177/1074248409343182. PMID 19721129. S2CID 44733401.
- ^ Unless else specified in boxes, then ref is: Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 978-0-443-07145-4.[page needed]
- ^ Kulmatycki KM, Abouchehade K, Sattari S, Jamali F (May 2001). "Drug-disease interactions: reduced beta-adrenergic and potassium channel antagonist activities of sotalol in the presence of acute and chronic inflammatory conditions in the rat". Br. J. Pharmacol. 133 (2): 286–94. doi:10.1038/sj.bjp.0704067. PMC 1572777. PMID 11350865.
- ^ Waller, Derek G.; Sampson, Tony (2013). Medical Pharmacology and Therapeutics E-Book. Elsevier Health Sciences. p. 144. ISBN 9780702055034.
- ^ "treatment of paroxysmal atrial fibrillation - General Practice Notebook". www.gpnotebook.co.uk.
- ^ "protocol for management of haemodynamically stable ventricular tachycardia – General Practice Notebook". www.gpnotebook.co.uk. Retrieved 2016-02-09.
- ^ Milne JR, Hellestrand KJ, Bexton RS, Burnett PJ, Debbas NM, Camm AJ (February 1984). "Class 1 antiarrhythmic drugs – characteristic electrocardiographic differences when assessed by atrial and ventricular pacing". Eur. Heart J. 5 (2): 99–107. doi:10.1093/oxfordjournals.eurheartj.a061633. PMID 6723689.
- ^ Trevor, Anthony J.; Katzung, Bertram G. (2003). Pharmacology. New York: Lange Medical Books/McGraw-Hill, Medical Publishing Division. p. 43. ISBN 978-0-07-139930-2.
- ^ Lenz, TL; Hilleman, DE (2000). "Dofetilide, a New Class III Antiarrhythmic Agent". Pharmacotherapy. 20 (7): 776–86. doi:10.1592/phco.20.9.776.35208. PMID 10907968.
- ^ Conti JB, Belardinelli L, Utterback DB, Curtis AB (March 1995). "Endogenous adenosine is an antiarrhythmic agent". Circulation. 91 (6): 1761–67. doi:10.1161/01.cir.91.6.1761. PMID 7882485.
- ^ Brugada P (July 2000). "Magnesium: an antiarrhythmic drug, but only against very specific arrhythmias". Eur. Heart J. 21 (14): 1116. doi:10.1053/euhj.2000.2142. PMID 10924290.
- ^ Hoshino K, Ogawa K, Hishitani T, Isobe T, Eto Y (October 2004). "Optimal administration dosage of magnesium sulfate for torsades de pointes in children with long QT syndrome". J Am Coll Nutr. 23 (5): 497S–500S. doi:10.1080/07315724.2004.10719388. PMID 15466950. S2CID 30146333.
- ^ Hoshino K, Ogawa K, Hishitani T, Isobe T, Etoh Y (April 2006). "Successful uses of magnesium sulfate for torsades de pointes in children with long QT syndrome". Pediatr Int. 48 (2): 112–17. doi:10.1111/j.1442-200X.2006.02177.x. PMID 16635167.
- ^ Lindberg JS, Zobitz MM, Poindexter JR, Pak CY (1990). "Magnesium bioavailability from magnesium citrate and magnesium oxide". Journal of the American College of Nutrition. 9 (1): 48–55. doi:10.1080/07315724.1990.10720349. PMID 2407766.
- ^ Charlier, R; Deltour, G; Baudine, A; Chaillet, F (November 1968). "Pharmacology of amiodarone, and anti-anginal drug with a new biological profile". Arzneimittel-Forschung. 18 (11): 1408–17. PMID 5755904.
- ^ Singh, BN; Vaughan Williams, EM (August 1970). "The effect of amiodarone, a new anti-anginal drug, on cardiac muscle". British Journal of Pharmacology. 39 (4): 657–67. doi:10.1111/j.1476-5381.1970.tb09891.x. PMC 1702721. PMID 5485142.
- ^ Damato, Anthony N. (1 July 1969). "Diphenylhydantoin: Pharmacological and clinical use". Progress in Cardiovascular Diseases. 12 (1): 1–15. doi:10.1016/0033-0620(69)90032-2. PMID 5807584.
- ^ "The 'Sicilian Gambit'. A new approach to the classification of antiarrhythmic drugs based on their actions on arrhythmogenic mechanisms. The Task Force of the Working Group on Arrhythmias of the European Society of Cardiology". Eur. Heart J. 12 (10): 1112–31. October 1991. PMID 1723682.
- ^ Vaughan Williams EM (November 1992). "Classifying antiarrhythmic actions: by facts or speculation". J Clin Pharmacol. 32 (11): 964–77. doi:10.1002/j.1552-4604.1992.tb03797.x. PMID 1474169.
- ^ "Milestones in the Evolution of the Study of Arrhythmias". Retrieved 2008-07-31.[dead link]
- ^ Fogoros, Richard N. (1997). Antiarrhythmic drugs: a practical guide. Oxford: Blackwell Science. p. 49. ISBN 978-0-86542-532-3.
- ^ Lei, Ming; Wu, Lin; Terrar, Derek A.; Huang, Christopher L.-H. (23 October 2018). "Modernized Classification of Cardiac Antiarrhythmic Drugs". Circulation. 138 (17): 1879–1896. doi:10.1161/CIRCULATIONAHA.118.035455. PMID 30354657.
- Antiarrhythmic agents
- Cardiac electrophysiology


