Magnesium sulfate
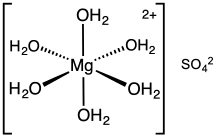 hexahydrate
| |
 Anhydrous magnesium sulfate
| |
 Epsomite (heptahydrate)
| |
| Names | |
|---|---|
| IUPAC name
Magnesium sulfate
| |
| Other names
Epsom salt (heptahydrate)
English salt Bitter salts Bath salts | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.028.453 |
| E number | E518 (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MgSO4 | |
| Molar mass | 120.366 g/mol (anhydrous) 138.38 g/mol (monohydrate) 174.41 g/mol (trihydrate) 210.44 g/mol (pentahydrate) 228.46 g/mol (hexahydrate) 246.47 g/mol (heptahydrate) |
| Appearance | white crystalline solid |
| Odor | odorless |
| Density | 2.66 g/cm3 (anhydrous) 2.445 g/cm3 (monohydrate) 1.68 g/cm3 (heptahydrate) 1.512 g/cm3 (11-hydrate) |
| Melting point | anhydrous decomposes at 1,124 °C monohydrate decomposes at 200 °C heptahydrate decomposes at 150 °C undecahydrate decomposes at 2 °C |
| anhydrous 26.9 g/100 mL (0 °C) 35.1 g/100 mL (20 °C) 50.2 g/100 mL (100 °C) heptahydrate 113 g/100 mL (20 °C) | |
| Solubility | 1.16 g/100 mL (18 °C, ether) slightly soluble in alcohol, glycerol insoluble in acetone |
| −50·10−6 cm3/mol | |
Refractive index (nD)
|
1.523 (monohydrate) 1.433 (heptahydrate) |
| Structure | |
| monoclinic (hydrate) | |
| Pharmacology | |
| A06AD04 (WHO) A12CC02 (WHO) B05XA05 (WHO) D11AX05 (WHO) V04CC02 (WHO) | |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | 
1
0
0 |
| Related compounds | |
Other cations
|
Beryllium sulfate Calcium sulfate Strontium sulfate Barium sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium sulfate or magnesium sulphate (in British English) is a chemical compound, a salt with the formula MgSO
4, consisting of magnesium cations Mg2+
(20.19% by mass) and sulfate anions SO2−
4. It is a white crystalline solid, soluble in water but not in ethanol.
Magnesium sulfate is usually encountered in the form of a hydrate MgSO
4·nH
2O, for various values of n between 1 and 11. The most common is the heptahydrate MgSO
4·7H
2O, known as Epsom salt, which is a household chemical with many traditional uses, including bath salts.[1]
The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient because of the role of magnesium in chlorophyll and photosynthesis). The monohydrate is favored for this use; by the mid 1970s, its production was 2.3 million tons per year.[2] The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs.
Hydrates[]
Magnesium sulfate can crystallize as several hydrates, including:
- Anhydrous, MgSO
4; unstable in nature, hydrates to form epsomite.[3] - Monohydrate, MgSO
4·H
2O; kieserite, monoclinic.[4] - MgSO
4·1.25H
2O or 8MgSO
4·10H
2O.[5] - Dihydrate, MgSO
4·2H
2O; orthorhombic. - MgSO
4·2.5H
2O or 2MgSO
4·5H
2O.[5] - Trihydrate, MgSO
4·3H
2O.[5] - Tetrahydrate, MgSO
4·4H
2O; , monoclinic.[6] - Pentahydrate, MgSO
4·5H
2O; , triclinic.[4] - Hexahydrate, MgSO
4·6H
2O; hexahydrite, monoclinic. - Heptahydrate, MgSO
4·7H
2O ("Epsom salt"); epsomite, orthorhombic.[4] - Enneahydrate, MgSO
4·9H
2O, monoclinic.[7] - Decahydrate, MgSO
4·10H
2O.[6] - Undecahydrate, MgSO
4·11H
2O; meridianiite, triclinic.[6]
As of 2017, the existence of the decahydrate apparently has not been confirmed.[7]
All the hydrates lose water upon heating. Above 320 °C, only the anhydrous form is stable. It decomposes without melting at 1124 °C into magnesium oxide (MgO) and sulfur trioxide (SO3).
Heptahydrate (Epsom salt)[]
The heptahydrate takes its common name "Epsom salt" from a bitter saline spring in Epsom in Surrey, England, where the salt was produced from the springs that arise where the porous chalk of the North Downs meets the impervious London clay.
The heptahydrate readily loses one equivalent of water to form the hexahydrate.
It is a natural source of both magnesium and sulphur. Epsom salts are commonly used in bath salts, exfoliants, muscle relaxers and pain relievers. However, these are different from Epsom salts that are used for gardening, as they contain aromas and perfumes not suitable for plants.[8]
Monohydrate[]
The monohydrate can be prepared by heating the hexahydrate to approximately 150 °C.[citation needed] Further heating to approximately 300–320 °C gives anhydrous magnesium sulfate.[citation needed]
Undecahydrate[]
The undecahydrate MgSO
4·11H
2O, meridianiite, is stable at atmospheric pressure only below 2 °C. Above that temperature, it liquefies into a mix of solid heptahydrate and a saturated solution. It has an eutectic point with water at −3.9 °C and 17.3% (mass) of MgSO4.[5] Large crystals can be obtained from solutions of the proper concentration kept at 0 °C for a few days.[5]
At pressures of about 0.9 GPa and at 240 K, meridianiite decomposes into a mixture of ice VI and the enneahydrate MgSO
4·9H
2O.[7]
Enneahydrate[]
This section does not cite any sources. (April 2021) |
The enneahydrate MgSO
4·9H
2O was identified and characterized only recently, even though it seems easy to produce (by cooling a solution of MgSO
4 and sodium sulfate Na
2SO
4 in suitable proportions).
The structure is monoclinic, with unit-cell parameters at 250 K: a = 0.675 nm, b = 1.195 nm, c = 1.465 nm, β = 95.1°, V = 1.177 with Z = 4. The most probable space group is P21/c. also forms an enneahydrate MgSeO
4·9H
2O, but with a different crystal structure.[7]
Natural occurrence[]
As Mg2+
and SO2−
4 ions are respectively the second cation and the second anion present in seawater after Na+
and Cl−
, magnesium sulfates are common minerals in geological environments. Their occurrence is mostly connected with supergene processes. Some of them are also important constituents of evaporitic potassium-magnesium (K-Mg) salts deposits.
Bright spots observed by the Dawn Spacecraft in Occator Crater on the dwarf planet Ceres are most consistent with reflected light from magnesium sulfate hexahydrate.[9]
Almost all known mineralogical forms of MgSO4 are hydrates. Epsomite is the natural analogue of "Epsom salt". Meridianiite, MgSO4·11H2O, has been observed on the surface of frozen lakes and is thought to also occur on Mars. Hexahydrite is the next lower (6) hydrate. Three next lower hydrates—, , and especially —are rare. Kieserite is a monohydrate and is common among evaporitic deposits. Anhydrous magnesium sulfate was reported from some burning coal dumps.
Preparation[]
Magnesium sulfate is usually obtained directly from dry lake beds and other natural sources. It can also be prepared by reacting magnesite (magnesium carbonate, MgCO
3) or magnesia (oxide, MgO) with sulfuric acid.
Another possible method is to treat seawater or magnesium-containing industrial wastes so as to precipitate magnesium hydroxide and react the precipitate with sulfuric acid.
Physical properties[]
Magnesium sulfate relaxation is the primary mechanism that causes the absorption of sound in seawater at frequencies above 10 kHz[10] (acoustic energy is converted to thermal energy). Lower frequencies are less absorbed by the salt, so that low frequency sound travels farther in the ocean. Boric acid and magnesium carbonate also contribute to absorption.[11]
Uses[]
Medical[]
Magnesium sulfate is used both externally (as Epsom salt) and internally.
The main external use is the formulation as bath salts, especially for foot baths to soothe sore feet. Such baths have been claimed to also soothe and hasten recovery from muscle pain, soreness, or injury.[12] Potential health effects of magnesium sulfate are reflected in medical studies on the impact of magnesium on resistant depression[13] and as an analgesic for migraine and chronic pain.[14] Magnesium sulfate has been studied in the treatment of asthma,[15] preeclampsia and eclampsia.[16]
Magnesium sulfate is the usual component of the concentrated salt solution used in isolation tanks to increase its specific gravity to approximately 1.25–1.26. This high density allows an individual to float effortlessly on the surface of water in the closed tank, eliminating as many of the external senses as possible.
In the UK, a medication containing magnesium sulfate and phenol, called "drawing paste", is useful for small boils or localized infections[17] and removing splinters.[18]
Internally, magnesium sulfate may be administered by oral, respiratory, or intravenous routes. Internal uses include replacement therapy for magnesium deficiency,[19] treatment of acute and severe arrhythmias,[20] as a bronchodilator in the treatment of asthma,[21] and preventing eclampsia.[22]
Agriculture[]
In agriculture, magnesium sulfate is used to increase magnesium or sulfur content in soil. It is most commonly applied to potted plants, or to magnesium-hungry crops such as potatoes, tomatoes, carrots, peppers, lemons, and roses. The advantage of magnesium sulfate over other magnesium soil amendments (such as dolomitic lime) is its high solubility, which also allows the option of foliar feeding. Solutions of magnesium sulfate are also nearly pH neutral, compared with the slightly alkaline salts of magnesium as found in limestone; therefore, the use of magnesium sulfate as a magnesium source for soil does not significantly change the soil pH.[23]
Magnesium sulfate was historically used as a treatment for lead poisoning prior to the development of chelation therapy, as it was hoped that any lead ingested would be precipitated out by the magnesium sulfate and subsequently purged from the digestive system.[24] This application saw particularly widespread use among veterinarians during the early-to-mid 20th century; Epsom salt was already available on many farms for agricultural use, and it was often prescribed in the treatment of farm animals that inadvertently ingested lead.[25][26]
Food preparation[]
Magnesium sulfate is used as a brewing salt in making beer.[27] It may also be used as a coagulant for making tofu.[28]
Chemistry[]
Anhydrous magnesium sulfate is commonly used as a desiccant in organic synthesis owing to its affinity for water and compatibility with most organic compounds. During work-up, an organic phase is treated with anhydrous magnesium sulfate. The hydrated solid is then removed by filtration, decantation, or by distillation (if the boiling point is low enough). Other inorganic sulfate salts such as sodium sulfate and calcium sulfate may be used in the same way.
Construction[]
Magnesium sulfate is used to prepare specific cements by the reaction between magnesium oxide and magnesium sulfate solution, which are of good binding ability and more resistance than Portland cement. This cement is mainly adopted in the production of lightweight insulation panels. Weakness in water resistance limits its usage.
Magnesium (or sodium) sulfate is also used for testing aggregates for soundness in accordance with ASTM C88 standard, when there are no service records of the material exposed to actual weathering conditions. The test is accomplished by repeated immersion in saturated solutions followed by oven drying to dehydrate the salt precipitated in permeable pore spaces. The internal expansive force, derived from the rehydration of the salt upon re-immersion, simulates the expansion of water on freezing.
Magnesium sulfate is also used to test the resistance of concrete to external sulfate attack (ESA).
Aquaria[]
Magnesium sulfate heptahydrate is also used to maintain the magnesium concentration in marine aquaria which contain large amounts of stony corals, as it is slowly depleted in their calcification process. In a magnesium-deficient marine aquarium, calcium and alkalinity concentrations are very difficult to control because not enough magnesium is present to stabilize these ions in the saltwater and prevent their spontaneous precipitation into calcium carbonate.[29]
Double salts[]
Double salts containing magnesium sulfate exist. There are several known as sodium magnesium sulfates and potassium magnesium sulfates. A mixed copper-magnesium sulfate heptahydrate (Mg,Cu)SO4·7H2O was recently found to occur in mine tailings and has been given the mineral name alpersite.[30]
See also[]
References[]
- ^ "Quick Cures/Quack Cures: Is Epsom Worth Its Salt?". The Wall Street Journal. 9 April 2012. Archived from the original on 12 April 2012. Retrieved 15 June 2019.
- ^ Industrial Inorganic Chemistry, Karl Heinz Büchel, Hans-Heinrich Moretto, Dietmar Werner, John Wiley & Sons, 2d edition, 2000, ISBN 978-3-527-61333-5
- ^ https://www.mindat.org/min-40082.html
- ^ a b c Odochian, Lucia (1995). "Study of the nature of the crystallization water in some magnesium hydrates by thermal methods". Journal of Thermal Analysis and Calorimetry. 45 (6): 1437–1448. doi:10.1007/BF02547437. Archived from the original on 26 August 2011. Retrieved 7 August 2010.
- ^ a b c d e A. Dominic Fortes, Frank Browning, and Ian G. Wood (2012): "Cation substitution in synthetic meridianiite (MgSO4·11H2O) I: X-ray powder diffraction analysis of quenched polycrystalline aggregates". Physics and Chemistry of Minerals, volume 39, issue, pages 419–441. doi:10.1007/s00269-012-0497-9
- ^ a b c R. C. Peterson, W. Nelson, B. Madu, and H. F. Shurvell (2007): "Meridianiite: A new mineral species observed on Earth and predicted to exist on Mars". American Mineralogist, volume 92, issue 10, pages 1756–1759. doi:10.2138/am.2007.2668
- ^ a b c d A. Dominic Fortes, Kevin S. Knight, and Ian G. Wood (2017): "Structure, thermal expansion and incompressibility of MgSO4·9H2O, its relationship to meridianiite (MgSO4·11H2O) and possible natural occurrences". Acta Crystallographica Section B: Structureal Science, Crystal Engineering and Materials, volume 73, part 1, pages 47-64. doi:10.1107/S2052520616018266
- ^ "What Is Epsom Salt And Why Is It So Important For My Cannabis Garden?". Herbies. Retrieved 28 October 2020.
- ^ M. C. De Sanctis; E. Ammannito; A. Raponi; S. Marchi; T. B. McCord; H. Y. McSween; F. Capaccioni; M. T. Capria; F. G. Carrozzo; M. Ciarniello; A. Longobardo; F. Tosi; S. Fonte; M. Formisano; A. Frigeri; M. Giardino; G. Magni; E. Palomba; D. Turrini; F. Zambon; J.-P. Combe; W. Feldman; R. Jaumann; L. A. McFadden; C. M. Pieters (2015). "Ammoniated phyllosilicates with a likely outer Solar System origin on (1) Ceres" (PDF). Nature. 528 (7581): 241–244. doi:10.1038/nature16172. PMID 26659184.
- ^ "Underlying physics and mechanisms for the absorption of sound in seawater". Resource.npl.co.uk. Archived from the original on 18 June 2009. Retrieved 6 July 2009.
- ^ Michael A. Ainslie, Principles of Sonar Performance Modeling, p.18
- ^ Ingraham, Paul. "Does Epsom Salt Work? The science of Epsom salt bathing for recovery from muscle pain, soreness, or injury". Pain Science. Archived from the original on 10 September 2016. Retrieved 29 August 2016.
- ^ Eby, George A.; Eby, Karen L. (April 2010). "Magnesium for treatment-resistant depression: a review and hypothesis". Medical Hypotheses. 74 (4): 649–660. doi:10.1016/j.mehy.2009.10.051. ISSN 1532-2777. PMID 19944540.
- ^ Banerjee, Srabani; Jones, Sarah (2017). Magnesium as an Alternative or Adjunct to Opioids for Migraine and Chronic Pain: A Review of the Clinical Effectiveness and Guidelines. CADTH Rapid Response Reports. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. PMID 29334449.
- ^ "Magnesium sulfate asthma – Search Results – PubMed". PubMed. Retrieved 29 June 2021.
- ^ "Magnesium sulfate eclampsia – Search Results – PubMed". PubMed. Retrieved 29 June 2021.
- ^ "Boots Magnesium Sulfate Paste B.P. - Patient Information Leaflet (PIL) - (eMC)". www.medicines.org.uk. Retrieved 14 April 2018.
- ^ "Removing a splinter with Magnesium Sulphate".
- ^ "Pharmaceutical Information – Magnesium Sulfate". RxMed. Archived from the original on 3 April 2009. Retrieved 6 July 2009.
- ^ "CPR and First Aid: Antiarrhythmic Drugs During and Immediately After Cardiac Arrest (section)". American Heart Association. Retrieved 29 August 2016.
Previous ACLS guidelines addressed the use of magnesium in cardiac arrest with polymorphic ventricular tachycardia (ie, torsades de pointes) or suspected hypomagnesemia, and this has not been reevaluated in the 2015 Guidelines Update. These previous guidelines recommended defibrillation for termination of polymorphic VT (ie, torsades de pointes), followed by consideration of intravenous magnesium sulfate when secondary to a long QT interval.
- ^ Blitz M, Blitz S, Hughes R, Diner B, Beasley R, Knopp J, Rowe BH (2005). "Aerosolized magnesium sulfate for acute asthma: a systematic review". Chest. 128 (1): 337–344. doi:10.1378/chest.128.1.337. PMID 16002955..
- ^ Duley, L; Gülmezoglu, AM; Henderson-Smart, DJ; Chou, D (10 November 2010). "Magnesium sulphate and other anticonvulsants for women with pre-eclampsia". The Cochrane Database of Systematic Reviews (11): CD000025. doi:10.1002/14651858.CD000025.pub2. PMC 7061250. PMID 21069663.
- ^ "Pubchem: magnesium sulfate". Archived from the original on 18 October 2016.
- ^ Wood, H. C. (1877). A Treatise on Therapeutics, Comprising Materia Medica and Toxicology, with Especial Reference to the Application of the Physiological Action of Drugs to Clinical Medicine. Philadelphia: J. B. Lippincott & Co. p. 34.
The treatment of acute lead-poisoning consists in the evacuation of the stomach, if necessary, the exhibition of the sulphate of sodium or of magnesium, and the meeting of the indications as they arrive. The Epsom and Glauber's salts act as chemical antidotes, by precipitating the insoluble sulphate of lead, and also, if in excess, empty the bowel of the compound formed.
- ^ Barker, C. A. V. (January 1945). "Experience with Lead Poisoning". Canadian Journal of Comparative Medicine and Veterinary Science. 9 (1): 6–8. PMC 1660962. PMID 17648099.
Udall (1) suggests sodium citrate as of some value together with Epsom salts which will bring about a precipitation of the lead in the form of an insoluble compound. Nelson (3) reported a case that survived following the use of a 20% magnesium sulphate solution intravenously, subcutaneously and orally. McIntosh (5) has suggested that purgative doses of Epsom salts may be effective in combining with the lead and overcoming the toxicity.
- ^ Herriot, James (1972). All Creatures Great and Small. New York: St. Martin's Press. p. 157. ISBN 0-312-08498-6.
The specific antidotes to metal poisoning had not been discovered and the only thing which sometimes did a bit of good was magnesium sulphate which caused the precipitation of insoluble lead sulphate. The homely term for magnesium sulphate is, of course, epsom salts.
- ^ "Magnesium Sulphate". National Home Brew. Archived from the original on 1 August 2016. Retrieved 4 January 2019.
- ^ US The present invention relates to a novel process for producing packed tofu, particularly a process for producing long-life packed tofu from sterilized soybean milk. 6042851, Matsuura, Masaru; Sasaki, Masaoki & Sasakib, Jun et al., "Process for producing packed tofu"
- ^ "Do-It-Yourself Magnesium Supplements for the Reef Aquarium". Reefkeeping. 2006. Archived from the original on 22 March 2008. Retrieved 14 March 2008.
- ^ Peterson, Ronald C.; Hammarstrom, Jane M.; Seal, II, Robert R (February 2006). "Alpersite (Mg,Cu)SO4·7H2O, a new mineral of the melanterite group, and cuprian pentahydrite: Their occurrence within mine waste". American Mineralogist. 91 (2–3): 261–269. doi:10.2138/am.2006.1911.
External links[]
- Desiccants
- Laxatives
- Magnesium compounds
- Sulfates