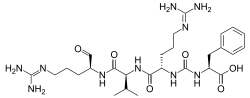
Antipain
| |
| Names | |
|---|---|
| IUPAC name
N2-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}-N5-(diaminomethylidene)-L-ornithyl-N-{(2S)-5-[(diaminomethylidene)amino]-1-oxopentan-2-yl}-L-valinamide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H44N10O6 | |
| Molar mass | 604.713 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain.[1] It was discovered in 1972 and was the first natural peptide found that contained an group.[2] Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases.[3]
It has been crystallised in complexes with carboxypeptidase, which is obtained from wheat,[4] and Leishmania major oligopeptidase B.[5] In both cases, the backbone carbonyl of the terminal arginine of antipain forms a covalent bond to the active site serine in the protease.
In oncology, pain control is an ongoing problem. An important obstacle to controlling cancer pain is related to the patient.[6] A recent research article indicated that antipain Y is a new type of anti-pain analog, which can inhibit the release of neurotransmitter in rat dorsal root ganglion neurons.[7] An experiment focused on potential improvement for pain management of oncology patients with antipain.[8]
A study was performed for information on the effect of antipain on the quality of post-thawed ram semen.[9] The results from this experiment concluded that antipain aided in the quality of ram semen by maintaining the sperm mobility.[10] Antipain includes the function to inhibit a degrading enzyme, called plasmin, permitting this substance to be able to improve the resistance of membrane disruption by freezing temperatures.[10]
Antiretroviral and protease inhibitors[]
There are several serine proteases, which are enzymes that cleave the protein bond, in the human genome. Proteases are ubiquitous. Protease function is also affected by endogenous inhibitors.[11] The abnormal functioning of these proteases can lead to the development of cancerous tumors.[12][13] Protease inhibitors or antipain are enzymes that are used to regulate their performance.
The antiretroviral drug Nelfinavir is one example of an antipain. It was classified as an antipain after a study published by Ovid that investigated the in vitro effect of Nelfinavir using proteolytic foot printing and found that it selectively inhibited HER2- positive, a growth factor in breast cancer.[14]
Antipain is a reversible inhibitor of trypsin, papain, and, plasmin, which is isolated from actinomycetes.[15]
Protease inhibitors and DRUG neurons[]
Protease-activated receptors (PARs) are a unique class of G protein-coupled receptors activated by proteolytic cleavage of the receptor N terminus.[16] PARs are activated by some Serine Proteases and are important for the physiological, psychological,[17] and pathological functions of the human body.
During the study, an antipain analogue Y was developed and studied. It was shown to have properties as a protease inhibitors but it had a low IC50 than a antipain. Antipain analogue Y was able to suppress Trypsin, which inhibits the secretion of an excitatory neuropeptide that leads to inflammation and other disorders.[citation needed] Antipain is a protease inhibitor, usually 1–2 μg/mL, and is well-against to cathepsin A, cathepsin B, papain and trypsin protease enzymes.[18]
References[]
- ^ Suda H, Aoyagi T, Hamada M, Takeuchi T, Umezawa H (April 1972). "Antipain, a new protease inhibitor isolated from actinomycetes". The Journal of Antibiotics. 25 (4): 263–266. doi:10.7164/antibiotics.25.263. PMID 4559651.
- ^ Umezawa S, Tatsuta K, Fujimoto K, Tsuchiya T, Umezawa H (April 1972). "Structure of antipain, a new Sakaguchi-positive product of streptomyces". The Journal of Antibiotics. 25 (4): 267–270. doi:10.7164/antibiotics.25.267. PMID 5052959.
- ^ Lackie J (2012). A Dictionary of Biomedicine. Oxford University Press. ISBN 9780199549351.
- ^ PDB ENTRY 1bcr Bullock TL, Breddam K, Remington SJ (February 1996). "Peptide aldehyde complexes with wheat serine carboxypeptidase II: implications for the catalytic mechanism and substrate specificity". Journal of Molecular Biology. 255 (5): 714–725. doi:10.1006/jmbi.1996.0058. PMID 8636973.
- ^ PDB ENTRY 2xe4 McLuskey K, Paterson NG, Bland ND, Isaacs NW, Mottram JC (December 2010). "Crystal structure of Leishmania major oligopeptidase B gives insight into the enzymatic properties of a trypanosomatid virulence factor". The Journal of Biological Chemistry. 285 (50): 39249–39259. doi:10.1074/jbc.M110.156679. PMC 2998157. PMID 20926390.
- ^ Koller A, Gaertner J, De Geest S, Hasemann M, Becker G (September 2018). "Testing the Implementation of a Pain Self-management Support Intervention for Oncology Patients in Clinical Practice: A Randomized Controlled Pilot Study (ANtiPain)". Cancer Nursing. 41 (5): 367–378. doi:10.1097/ncc.0000000000000502. PMID 28537957.
- ^ Nakae K, Kojima F, Sawa R, Kubota Y, Igarashi M, Kinoshita N, et al. (January 2010). "Antipain Y, a new antipain analog that inhibits neurotransmitter release from rat dorsal root ganglion neurons". The Journal of Antibiotics. 63 (1): 41–44. doi:10.1038/ja.2009.109. PMID 19911027.
- ^ Koller A (2018). Testing the Implementation of a Pain Self-management Support Intervention for Oncology Patients in Clinical Practice: A Randomized Controlled Pilot Study (ANtiPain). Wolters Kluwer Health, Inc. pp. 1–18.
- ^ "The protease inhibitor antipain has a beneficial synergistic effect with trehalose for ram semen cryopreservation".
- ^ a b The Protease Inhibitor Antipain has a Beneficial Synergistic Effect with Trehalose for Ram Semen Cryopreservation. Germany: Wiley Subscription Services, Inc. 2018. pp. 2–42.
- ^ Dhalla NS (2014). Role of Proteases in Cellular Dysfunction. New York, NY : Springer New York : Imprint: Springer. pp. 3–15.
- ^ Quesada V, Ordóñez GR, Sánchez LM, Puente XS, López-Otín C (January 2009). "The Degradome database: mammalian proteases and diseases of proteolysis". Nucleic Acids Research. 37 (Database issue): D239–D243. doi:10.1093/nar/gkn570. PMC 2686449. PMID 18776217.
- ^ Mariño G, Uría JA, Puente XS, Quesada V, Bordallo J, López-Otín C (February 2003). "Human autophagins, a family of cysteine proteinases potentially implicated in cell degradation by autophagy". The Journal of Biological Chemistry. 278 (6): 3671–3678. doi:10.1074/jbc.M208247200. PMID 12446702.
- ^ Shim JS, Rao R, Beebe K, Neckers L, Han I, Nahta R, Liu JO (October 2012). "Selective inhibition of HER2-positive breast cancer cells by the HIV protease inhibitor nelfinavir". Journal of the National Cancer Institute. 104 (20): 1576–1590. doi:10.1093/jnci/djs396. PMC 3472971. PMID 23042933.
- ^ "Antipain", Encyclopedic Dictionary of Genetics, Genomics and Proteomics, Hoboken, NJ, USA: John Wiley & Sons, Inc., 2004-07-15, ISBN 0-471-68422-8, retrieved 2021-03-29
- ^ Nakae K, Kojima F, Sawa R, Kubota Y, Igarashi M, Kinoshita N, et al. (January 2010). "Antipain Y, a new antipain analog that inhibits neurotransmitter release from rat dorsal root ganglion neurons". The Journal of Antibiotics. 63 (1): 41–44. doi:10.1038/ja.2009.109. PMID 19911027.
- ^ Nakae K, Saito K, Iino T, Yamamoto N, Wakabayashi M, Yoshikawa S, et al. (December 2005). "A prostacyclin receptor antagonist inhibits the sensitized release of substance P from rat sensory neurons". The Journal of Pharmacology and Experimental Therapeutics. 315 (3): 1136–1142. doi:10.1124/jpet.105.091967. PMID 16109742. S2CID 14841421.
- ^ "Antipain", Encyclopedia of Genetics, Genomics, Proteomics and Informatics, Dordrecht: Springer Netherlands, pp. 120–120, 2008, ISBN 978-1-4020-6753-2, retrieved 2021-03-29
- Peptides
- Aldehydes
- Biochemistry stubs