Auwers synthesis
| Auwers synthesis | |
|---|---|
| Named after | Karl von Auwers |
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000474 |
The Auwers synthesis is a series of organic reactions forming a flavonol from a coumarone. This reaction was first reported by Karl von Auwers in 1908.[1][2][3][4][5]

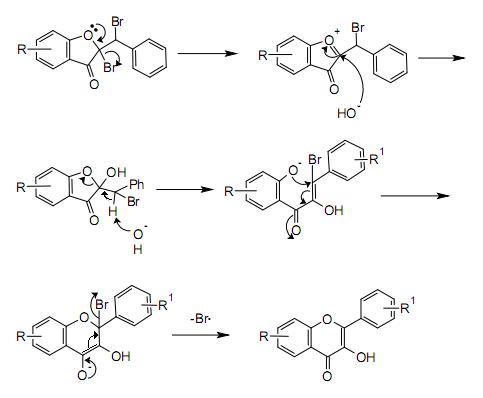
The first step in this procedure is an acid catalyzed aldol condensation between benzaldehyde and a 3-cyclooxapentanone to an o-hydroxychalcone. Bromination of the alkene group gives a dibromo-adduct which rearranges to the flavonol by reaction with potassium hydroxide.
Mechanism[]
A possible mechanism for the rearrangement step is shown below:
See also[]
References[]
- ^ K. Auwers, K. Müller, "Umwandlung von Benzal-cumaranonen in Flavonole", Ber. Dtsch. Chem. Ges., 41, 4233–4241 (1908) (doi:10.1002/cber.190804103137).
- ^ K. v. Auwers, P. Pohl, "Über die Umwandlung von Benzalcumaranonen in Flavonole", Liebigs Ann. Chem., 405, 243–294 (1914) (doi:10.1002/jlac.19144050302).
- ^ K. v. Auwers, P. Pohl, "Eine Synthese des Fisetins", Ber. Dtsch. Chem. Ges., 48, 85–90 (1915) (doi:10.1002/cber.19150480114).
- ^ K. v. Auwers, "Zur Bildung von Flavonolen aus Benzal-cumaranonen", Ber. Dtsch. Chem. Ges., 49, 809–819 (1916) (doi:10.1002/cber.19160490188).
- ^ K. v. Auwers, E. Auffenberg, "Über Cumaranone und Hydrindone", Ber. Dtsch. Chem. Ges., 52, 92-113 (1919) (doi:10.1002/cber.19190520114).
Categories:
- Coupling reactions
- Heterocycle forming reactions
- Name reactions
- Oxygen heterocycle forming reactions
- Ring expansion reactions
- Carbon-carbon bond forming reactions