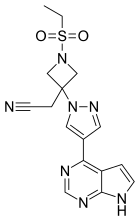
Baricitinib
| |||
| Clinical data | |||
|---|---|---|---|
| Trade names | Olumiant, others | ||
| Other names | INCB28050, LY3009104 | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a618033 | ||
| License data |
| ||
| Pregnancy category | |||
| Routes of administration | By mouth (tablets) | ||
| ATC code | |||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Bioavailability | 79% | ||
| Protein binding | 50% | ||
| Metabolism | CYP3A4 (<10%) | ||
| Elimination half-life | 12.5 hours | ||
| Excretion | 75% urine, 20% faeces | ||
| Identifiers | |||
| CAS Number | |||
| PubChem CID | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| PDB ligand | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.219.080 | ||
| Chemical and physical data | |||
| Formula | C16H17N7O2S | ||
| Molar mass | 371.42 g·mol−1 | ||
| 3D model (JSmol) | |||
Baricitinib, sold under the brand name Olumiant among others, is a drug for the treatment of rheumatoid arthritis (RA) in adults whose disease was not well controlled by tumor necrosis factor (TNF) inhibitors.[6] It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2.[7] The drug is approved for medical use in the European Union[5] and in the United States.[6][8]An important side effect of Jakinibs is serious bacterial, mycobacterial, fungal and viral infections.[9]
Medical uses[]
In February 2017, baricitinib was approved for use in the EU as a second-line therapy for moderate to severe active rheumatoid arthritis in adults, either alone or in combination with methotrexate.[10][5]
On 31 May 2018, the FDA approved barictinib for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more TNF antagonist therapies.[8][6][4]
Contraindications[]
During pregnancy, the use of baricitinib is contraindicated.[2][10]
Side effects[]
In studies, upper respiratory tract infections and high blood cholesterol levels (hypercholesterolemia) occurred in more than 10% of patients. Less common side effects included other infections such as herpes zoster, herpes simplex, urinary tract infections, and gastroenteritis.[10]
Interactions[]
Being metabolized only to a small extent, the substance has a low potential for interactions. In studies, inhibitors of the liver enzymes CYP3A4, CYP2C19, and CYP2C9, as well as the CYP3A4 inducer rifampicin, had no relevant influence on baricitinib concentrations in the bloodstream. While baricitinib blocks a number of transporter proteins in vitro, clinically relevant interactions via this mechanism are considered very unlikely, except perhaps for the cation transporter SLC22A1 (OCT1).[10]
An additive effect with other immunosuppressants cannot be excluded.[10]
Pharmacology[]
Mechanism of action[]
Baricitinib is a Janus kinase (JAK) inhibitor that reversibly inhibits Janus kinase 1 with a half maximal inhibitory concentration (IC50) of 5.9 nM and Janus kinase 2 with an IC50 of 5.7 nM. Tyrosine kinase 2, which belongs to the same enzyme family, is affected less (IC50 = 53 nM), and Janus kinase 3 far less (IC50 > 400 nM). Via a signal transduction pathway involving STAT proteins, this ultimately modulates gene expression in immunological cells.[10]
Other JAK inhibitors include tofacitinib, which is indicated for the treatment of rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis;[11][12] fedratinib,[13] and ruxolitinib.[14][15]
Pharmacokinetics[]
The substance is quickly absorbed from the gut with an absolute bioavailability of 79%. It reaches highest blood plasma levels after about an hour; in different individuals the time to reach this level ranges from 0.5 to 3 hours. Food intake has no relevant influence on the drug's pharmacokinetics. 50% of the circulating baricitinib are bound to blood plasma proteins.[10]
Less than 10% of the substance is metabolized to four different oxidation products by CYP3A4; the rest is left unchanged. Elimination half-life is 12.5 hours on average. About 75% is eliminated via the urine, and 20% via the faeces.[10]
History[]
As of August 2016, 31 clinical trials had been registered for baricitinib of which 24 had completed,[16] and 4 of 6 phase 3 trials had completed.[17][needs update]
COVID-19[]
In April 2020, Lilly announced they are investigating the use of baricitinib for treating people with COVID-19. The drug's anti-inflammatory activity is expected to act on the inflammatory cascade associated with COVID-19.[18]
In November 2020, published research showed barcitinib was beneficial in treating people with COVID-19. According to the paper "mechanistic actions of a Janus kinase-1/2 inhibitor targeting viral entry, replication and the cytokine storm, and is associated with beneficial outcomes including in severely ill elderly people".[19]
In a clinical trial of hospitalized people with COVID-19, baricitinib, in combination with remdesivir, was shown to reduce time to recovery within 29 days after initiating treatment compared to participants who received a placebo with remdesivir.[20] The safety and effectiveness of this investigational therapy for use in the treatment of COVID-19 continues to be evaluated.[20]
The data supporting the US Food and Drug Administration (FDA) emergency use authorization (EUA) for baricitinib combined with remdesivir are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), which was conducted by the US National Institute of Allergy and Infectious Diseases (NIAID).[20][21] This clinical trial evaluated whether baricitinib impacted how long it took for subjects who were also taking remdesivir to recover from COVID-19.[20] The trial followed participants for 29 days and included 1,033 participants with moderate or severe COVID-19; 515 participants received baricitinib plus remdesivir, and 518 participants received placebo plus remdesivir.[20] Recovery was defined as either being discharged from the hospital or being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care.[20] The median time to recovery from COVID-19 was seven days for baricitinib plus remdesivir and eight days for placebo plus remdesivir.[20] The odds of a patient's condition progressing to death or being ventilated at day 29 was lower in the baricitinib plus remdesivir group versus the placebo plus remdesivir group.[20] The odds of clinical improvement at day 15 was higher in the baricitinib plus remdesivir group versus the placebo plus remdesivir group.[20] For all of these endpoints, the effects were statistically significant.[20] The EUA was issued to Eli Lilly and Company.[20]
In November 2020, the World Health Organization (WHO) updated its guideline on therapeutics for COVID-19 to include a conditional recommendation against the use of remdesivir, triggered by results from the WHO Solidarity trial.[22][23]
In November 2020, the FDA issued an emergency use authorization (EUA) for the combination of baricitinib with remdesivir, for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized people aged two years of age or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).[24][25][20]
In July 2021, the FDA revised the EUA for baricitinib now authorizing it alone for the treatment of COVID-19 in hospitalized people aged two years of age or older requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).[24][25][26] Under the revised EUA, baricitinib is no longer required to be administered with remdesivir.[26]
Society and culture[]
Legal status[]
In January 2016, Eli Lilly submitted a new drug application to the US Food and Drug Administration (FDA) for the approval of baricitinib to treat moderately-to-severely active rheumatoid arthritis.[27]
In December 2016, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the approval of baricitinib as a therapy for rheumatoid arthritis.[7] European Union approval was granted in February 2017.[5]
Despite widespread expectations that the FDA would approve baricitinib for rheumatoid arthritis,[28] in April 2017, the FDA issued a rejection, citing concerns about dosing and safety.[29][30]
In May 2018, baricitinib was approved in the United States for the treatment of rheumatoid arthritis.[6][8]
In March 2020, the US FDA granted breakthrough therapy designation to baricitinib for the treatment of alopecia areata.[31]
Brand names[]
In Bangladesh the drug is sold under the trade name Baricinix and Baricent (Incepta Pharma) among others.[citation needed]
References[]
- ^ Jump up to: a b "Olumiant Product Information" (PDF). Therapeutic Goods Administration (TGA). Retrieved 12 June 2021.
- ^ Jump up to: a b "Baricitinib (Olumiant) Use During Pregnancy". Drugs.com. 8 November 2019. Retrieved 16 March 2020.
- ^ "AusPAR: Baricitinib". Therapeutic Goods Administration (TGA). 20 May 2021. Retrieved 11 June 2021.
- ^ Jump up to: a b "Olumiant- baricitinib tablet, film coated". DailyMed. 13 November 2019. Retrieved 16 March 2020.
- ^ Jump up to: a b c d "Olumiant EPAR". European Medicines Agency (EMA). 3 December 2019. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Jump up to: a b c d "Drug Trials Snapshots: Olumiant". U.S. Food and Drug Administration (FDA). 31 May 2018. Retrieved 16 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Jump up to: a b "Summary of opinion for Olumiant" (PDF). European Medicines Agency (EMA). 15 December 2016.
- ^ Jump up to: a b c "Drug Approval Package: Olumiant (baricitinib)". U.S. Food and Drug Administration (FDA). 5 July 2018. Retrieved 16 March 2020.
- ^ O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A (April 2013). "Janus kinase inhibitors in autoimmune diseases". Annals of the Rheumatic Diseases. 72 (suppl 2): ii111-5. doi:10.1136/annrheumdis-2012-202576. PMC 3616338. PMID 23532440.
- ^ Jump up to: a b c d e f g h "Olumiant: EPAR – Product Information" (PDF). European Medicines Agency. 13 February 2017.
- ^ "Xeljanz- tofacitinib tablet, film coated Xeljanz XR- tofacitinib tablet, film coated, extended release". DailyMed. 20 December 2019. Retrieved 28 April 2020.
- ^ "FDA approves Xeljanz for rheumatoid arthritis" (Press release). 6 November 2012.
- ^ "Inrebic- fedratinib hydrochloride capsule". DailyMed. 16 August 2019. Retrieved 28 April 2020.
- ^ Mesa RA (June 2010). "Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis". IDrugs. 13 (6): 394–403. PMID 20506062.
- ^ "Jakafi- ruxolitinib tablet". DailyMed. 26 February 2020. Retrieved 28 April 2020.
- ^ "Baricitinib clinical trials". ClinicalTrials.gov.
- ^ "Baricitinib phase 3 clinical trials". ClinicalTrials.gov.
- ^ "Eli Lilly to study baricitinib for Covid-19 treatment". Clinical Trials Arena.
- ^ Stebbing J, Sánchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, et al. (January 2021). "JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality". Science Advances. 7 (1): eabe4724. Bibcode:2021SciA....7.4724S. doi:10.1126/sciadv.abe4724. PMC 7775747. PMID 33187978.
- ^ Jump up to: a b c d e f g h i j k l "Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19". U.S. Food and Drug Administration (FDA) (Press release). 19 November 2020. Retrieved 19 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Baricitinib Emergency Use Authorization (EUA) Center for Drug Evaluation and Research (CDER) Review" (PDF). U.S. Food and Drug Administration (FDA). November 2020.
- ^ World Health Organization (2021). Therapeutics and COVID-19: living guideline, 6 July 2021 (Report). World Health Organization (WHO). hdl:10665/342368. WHO/2019-nCoV/therapeutics/2021.2. Lay summary.
- ^ Rochwerg B, Agarwal A, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, et al. (September 2020). "A living WHO guideline on drugs for covid-19". BMJ. 370: m3379. doi:10.1136/bmj.m3379. PMID 32887691. Lay summary.
- ^ Jump up to: a b "Baricitinib Emergency Use Authorization (EUA)" (PDF). U.S. Food and Drug Administration (FDA). July 2021.
- ^ Jump up to: a b "Fact Sheet for Baricitinib Emergency Use Authorization (EUA)" (PDF). U.S. Food and Drug Administration (FDA). July 2021.
- ^ Jump up to: a b "Coronavirus (COVID-19) Update: July 30, 2021". U.S. Food and Drug Administration (FDA) (Press release). 30 July 2021. Retrieved 30 July 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Lilly and Incyte Announce Submission of NDA to FDA for Oral Once-Daily Baricitinib for Treatment of Moderate-to-Severe Rheumatoid Arthritis". Drugs.com. 19 January 2016.
- ^ Carroll J (13 April 2017). "We don't know when (exactly) Lilly will announce the FDA's baricitinib decision, but watch out for the looming pricing squabble". Endpoints News.
- ^ Ramsey L (17 April 2017). "The FDA shot down a new rheumatoid arthritis drug — and the companies that make the drug are tumbling". Business Insider.
- ^ Grant C (14 April 2017). "Surprise FDA Rejection Will Sting This Biotech". The Wall Street Journal.
- ^ "Lilly Receives FDA Breakthrough Therapy Designation for Baricitinib for the Treatment of Alopecia Areata" (Press release). Eli Lilly and Company. 16 March 2020. Retrieved 16 March 2020 – via PR Newswire.
Further reading[]
- Cingolani A, Tummolo AM, Montemurro G, Gremese E, Larosa L, Cipriani MC, et al. (October 2020). "Baricitinib as rescue therapy in a patient with COVID-19 with no complete response to sarilumab". Infection. 48 (5): 767–771. doi:10.1007/s15010-020-01476-7. PMC 7340855. PMID 32642806.
- Jorgensen SC, Tse CL, Burry L, Dresser LD (August 2020). "Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19". Pharmacotherapy. 40 (8): 843–856. doi:10.1002/phar.2438. PMC 7323235. PMID 32542785.
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. (March 2021). "Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19". The New England Journal of Medicine. 384 (9): 795–807. doi:10.1056/NEJMoa2031994. PMC 7745180. PMID 33306283.
- Seif F, Aazami H, Khoshmirsafa M, Kamali M, Mohsenzadegan M, Pornour M, Mansouri D (2020). "JAK Inhibition as a New Treatment Strategy for Patients with COVID-19". International Archives of Allergy and Immunology. 181 (6): 467–475. doi:10.1159/000508247. PMC 7270061. PMID 32392562.
- Stebbing J, Krishnan V, de Bono S, Ottaviani S, Casalini G, Richardson PJ, et al. (August 2020). "Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients". EMBO Molecular Medicine. 12 (8): e12697. doi:10.15252/emmm.202012697. PMC 7300657. PMID 32473600.
- Zhang X, Zhang Y, Qiao W, Zhang J, Qi Z (September 2020). "Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19". International Immunopharmacology. 86: 106749. doi:10.1016/j.intimp.2020.106749. PMC 7328558. PMID 32645632.
External links[]
- "Baricitinib". Drug Information Portal. U.S. National Library of Medicine.
- Anti-inflammatory agents
- Azetidines
- Breakthrough therapy
- Heterocyclic compounds with 1 ring
- Nitrogen heterocycles
- Non-receptor tyrosine kinase inhibitors
- Pyrazoles
- Pyrrolopyrimidines
- Sulfonamides