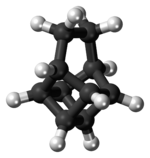
Basketane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pentacyclo[4.4.0.02,5.03,8.04,7]decane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H12 | |||
| Molar mass | 132.206 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently[1] by Masamune[2] and Dauben and Whalen.[3] A European patent published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio.[4]
Chemical Nomenclature[]
In the year 1989 and before the synthesis of basketane, historic chemists were intrigued by the structural make-up of molecules, specifically objects you see everyday.[5] Using supramolecular chemistry, molecules such as cubane and basketane were named according to their corresponding shape and historically revealed certain characteristics and personal motives of chemists at that time.[5] Naming these uniquely shaped molecules were also done considering chemical nomenclature such as adding "-anes" for single carbon-carbon bonds and "-enes" for double carbon-carbon bonds to the end of the appropriate molecules.[6]
Molecular Chemistry[]
Binmore et al.1994 synthesized basketane using cubane and creates this radical biproduct by forcing cubane rings to be opened up via structural strain to create the chemical bonds necessary for this rigid molecule.[7] This method is known as ring expansion where one part of two conjoined ringed are opened and rearranged to remove barriers between the two ring systems.[8] Cyclobutylmethyl radicals that rearrange and open into structures such as basketane and cubane are favorable rearrangements with free energy barriers around 0.3 kcal/mol.[8] Such polycyclic, cage-like molecules do not conform to simple carbon-carbon bonds with angles of 109.5 degrees due to their strained system.[9] The strain energy causes thermodynamic instability resulting in higher combustion and heat release.[9] When taking a mass analysis, the mass spectrum graph for basketane has a distinct tall peak at 39 m/z distinguishing a clear cyclic structure.[10]
Internet searches of basketane are saturated with entry level chemistry websites that use this and other molecules such has housane as intriguing fun facts on how unusual molecular structures can be.
See also[]
- Cubane
- 8-Aminopentacyclo class of 'Polycyclic cage molecules'
References[]
- ^ Marchand, A. P. (1989). "Synthesis and chemistry of homocubanes, bishomocubanes, and trishomocubanes". Chem. Rev. 89 (5): 1011–1033. doi:10.1021/cr00095a004.
- ^ Masamune, S.; Cuts, H.; Hogben, M. G. (1966). "Strained systems. VII. Pentacyclo[4.2.2.02,5.03,8.04,7]deca-9-ene, basketene". Tetrahedron Lett. 7 (10): 1017–1021. doi:10.1016/S0040-4039(00)70232-2.
- ^ Dauben, W. G.; Whalen, D. L. (1966). "Pentacyclo[4.4.0.02,5.03,8.04,7]decane and pentacyclo[4.3.0.02,5.03,8.04,7]nonane". Tetrahedron Lett. 7 (31): 3743–3750. doi:10.1016/S0040-4039(01)99958-7.
- ^ [1], "Process for depositing layers of diamond", issued 1987-09-08
- ^ a b Vicens, Jacques (2007-07-26). "Aesthetics in chemistry". Journal of Inclusion Phenomena and Macrocyclic Chemistry. 58 (3–4): 327–328. doi:10.1007/s10847-006-9161-7. ISSN 0923-0750. S2CID 94479873.
- ^ "2.3.2". www.nanomedicine.com. Retrieved 2021-02-23.
- ^ Binmore, Gavin T.; Della, Ernest W.; Elsey, Gordon M.; Head, Nicholas J.; Walton, John C. (April 1994). "Homolytic Reactions of Homocubane and Basketane: Rearrangement of the 9-Basketyl Radical by Multiple .beta.-Scissions". Journal of the American Chemical Society. 116 (7): 2759–2766. doi:10.1021/ja00086a009. ISSN 0002-7863.
- ^ a b "Ring Opening versus Ring Expansion in Rearrangement of Bicyclic Cyclobutylcarbinyl Radicals". dx.doi.org. doi:10.1021/jo702164r.s002. Retrieved 2021-03-16.
- ^ a b Marchand, Alan P. (1989-07-01). "Synthesis and chemistry of homocubanes, bishomocubanes, and trishomocubanes". Chemical Reviews. 89 (5): 1011–1033. doi:10.1021/cr00095a004. ISSN 0009-2665.
- ^ "Basketane - MS - Spectrum - SpectraBase". spectrabase.com. Retrieved 2021-03-16.
Further reading[]
- Binmore, Gavin T.; Della, Ernest W.; Elsey, G. M.; Head, N. J.; Walton, J. C. (1994). "Homolytic Reactions of Homocubane and Basketane: Rearrangement of the 9-Basketyl Radical by Multiple β-Scissions". J. Am. Chem. Soc. 116 (7): 2759–2766. doi:10.1021/ja00086a009.
- Cyclobutanes
- Hydrocarbon stubs