Housane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bicyclo[2.1.0]pentane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C5H8 | |||
| Molar mass | 68.119 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Boiling point | 45.5 ′C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
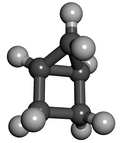
Housane or bicyclo[2.1.0]pentane is a saturated cycloalkane with the formula C5H8. It is a colorless volatile liquid at room temperature. It was named "housane" because of its shape. Structurally, the molecule consists of cyclopropane fused to cyclobutane. The synthesis of molecules containing multiple strained rings, such as housane, is a traditional endeavor in synthetic organic chemistry. This compound is prepared in several steps starting with cyclopentadiene.[1]
The two rings are fused in a cis configuration—this meso compound formally has (1R,4S) absolute stereochemistry. The small size of the two rings prevents the trans isomer from existing, so the stereochemistry is not usually mentioned when discussing this structure.
References[]
- ^ P. G. Gassman, K. T. Mansfield "Bicyclo[2.1.0]pentane" Org. Synth. 1969, volume 49, pp. 1. doi:10.15227/orgsyn.049.0001
Categories:
- Cyclobutanes
- Hydrocarbons
- Cyclopropanes
- Bicycloalkanes
- Hydrocarbon stubs