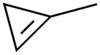
1-Methylcyclopropene
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Methylcycloprop-1-ene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | 1-MCP | ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.130.871 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| C4H6 | |||
| Molar mass | 54.09 g/mol | ||
| Boiling point | ~12 °C[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1-Methylcyclopropene (1-MCP) is a cyclopropene derivative used as a synthetic plant growth regulator. It is structurally related to the natural plant hormone ethylene and it is used commercially to slow down the ripening of fruit and to help maintain the freshness of cut flowers.[2]
Synthesis[]
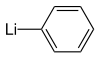
1-Methylcyclopropene is synthesized by the reaction of methallyl chloride and phenyllithium, which functions as a base:
The phenyllithium should be free of lithium halides. The corresponding reaction of allyl chloride and phenyllithium main affords cyclopropylbenzene.[3]
Isomers[]
Methylcyclopropene can refer to either of two isomers, 1-methylcyclopropene covered in this article, or 3-methylcyclopropene[4] which is not covered in this article. 2-methylcyclopropene would be an incorrect name for 1-methylcyclopropene. Also note: methylcyclopropane is yet a different chemical compound, which is a cycloalkane with the formula C4H8.
Mechanism of action[]
Ethylene is a gas acting at trace levels (typically between a few tenths and a few thousands ppm in the gas atmosphere) throughout the life of a plant by stimulating or regulating various processes such as the ripening of climacteric fruit, the opening of flowers (dehiscence process), and the shedding of leaves (abscission process).[5][6] The mechanism of action of 1-MCP involves its tightly binding to the ethylene receptor in plants, thereby blocking the effects of ethylene (competitive inhibitor).[7][8]
Commercial use[]

1-MCP is used commercially to maintain the freshness of ornamental plants and flowers and preventing the ripening of fruits. It is used in enclosed sites, such as coolers, truck trailers, greenhouses, storage facilities, and shipping containers.[9]
Under the brand name EthylBloc, 1-MCP was approved in 1999 by the U.S. Environmental Protection Agency for use on ornamental crops.[10] For cut flowers, potted flowers, and bedding, nursery and foliage plants, 1-MCP prevents or delays wilting, leaf yellowing, opening, and death.[11][12]
Under the brand name SmartFresh, 1-MCP is used in the agriculture industry by growers, packers, and shippers to prevent or delay the natural ripening process. The use of 1-MCP in agricultural products including apples, kiwifruit, tomatoes, bananas, plums, persimmons, avocados, and melons has been approved and accepted for use in more than 34 countries including the European Union and the United States.[13] Although benefiting from fresher produce and lower cost, the consumer however may be purchasing fruit that is older than expected.[14]
1-MCP is also being developed as a crop protection technique. By spraying 1-MCP on growing field crops during times of stress, the crops may be protected from moderate heat and drought conditions.[15]
See also[]
- Ethylene as a plant hormone
- Methylenecyclopropane, an isomer
References[]
- ^ Daly James and Kourelis Bob, January 25, 2000. Synthesis methods, complexes and delivery methods for the safe and convenient storage, transport and application of compounds for inhibiting the ethylene response in plants. US Patent 6,017,849.
- ^ Blankenship, Sylvia M; Dole, John M (April 2003). "1-Methylcyclopropene: a review". Postharvest Biology and Technology. 28 (1): 1–25. doi:10.1016/S0925-5214(02)00246-6.
- ^ Clarke, T. C.; Duncan, C. D.; Magid, R. M. (1971). "An Efficient and Convenient Synthesis of 1-Methylcyclopropene". J. Org. Chem. 36: 1320. doi:10.1021/jo00808a041.
- ^ Chem->Sink 3-methylcyclopropene
- ^ Chow B, McCourt P (2006). "Plant hormone receptors: perception is everything". Genes Dev. 20 (15): 1998–2008. doi:10.1101/gad.1432806. PMID 16882977.
- ^ De Paepe A, Van der Straeten D (2005). "Ethylene biosynthesis and signaling: an overview". Vitam Horm. Vitamins & Hormones. 72: 399–430. doi:10.1016/S0083-6729(05)72011-2. ISBN 978-0-12-709872-2. PMID 16492477.
- ^ Serek, M.; Tamari, G.; Sisler, E.C.; Borochov, A. (1995). "Inhibition of ethylene-induced cellular senescence symptoms by 1-methylcyclopropene, a new inhibitor of ethylene action". Physiol. Plant. 94 (2): 229–232. doi:10.1111/j.1399-3054.1995.tb05305.x.
- ^ Sisler E.C., Serek M. (2003). "Compounds interacting with the ethylene receptor in plants". Plant Biol. 5 (5): 473–80. doi:10.1055/s-2003-44782.
- ^ 1-Methylcyclopropene Fact Sheet, U.S. Environmental Protection Agency
- ^ Jim Daly; Anne Schluter (2001). "EthylBloc — An Industry Perspective" (PDF). Perishables Handling Quarterly (108): 5. Archived from the original (PDF) on 2010-06-13. Retrieved 2008-02-05.
- ^ What is EthylBloc technology? Archived 2008-01-30 at the Wayback Machine, at agrofresh.com
- ^ EthylBloc Ethylene Inhibitor, at floralife.com
- ^ SmartFresh Quality System Archived 2008-02-11 at the Wayback Machine at agrofresh.com
- ^ Europeans buying year-old apples Archived 2007-03-14 at the Wayback Machine, by Leah Vyse, December 13, 2005.
- ^ Syngenta-Agrofresh Alliance: New crop protection technology takes aim at row crops Archived 2008-06-26 at the Wayback Machine, Farm Industry News, Jan 18, 2008
| Wikimedia Commons has media related to 1-Methylcyclopropene. |
- Cyclopropenes
- Plant growth regulators