Bechamp reduction
The Bechamp reduction is used to reduce aromatic nitro compounds to their corresponding anilines, using iron and hydrochloric acid.[1]
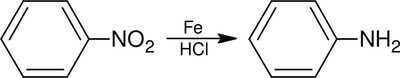
This reaction was originally used to produce large amounts of aniline for industry, but catalytic hydrogenation is the preferred method.[2] The Bechamp reaction is of interest as a route to iron oxide pigments.[3]
Reaction history and scope[]
The reaction was first used by Antoine Béchamp to reduce nitronaphthalene and nitrobenzene to naphthylamine and aniline, respectively.[4] The Bechamp reduction is broadly applicable to aromatic nitro compounds.[5][6][7]
Aliphatic nitro compounds are however more difficult to reduce, often remaining as the hydroxylamine. Tertiary aliphatic nitro compounds, however, are converted in good yield to the amine compound using the Bechamp reduction.[8]
Proposed mechanism[]
The reduction is thought to proceed in a multistep manner. First, the nitro group is reduced to a nitroso group, followed by a hydration reaction to a hydroxylamino group. Another reduction step then yields the amine.[9]

References[]
- ^ Org React 2, 428 (1944).
- ^ McKetta, John J. (1989). "Nitrobenzene and Nitrotoluene". Encyclopedia of Chemical Processing and Design: Volume 31 - Natural Gas Liquids and Natural Gasoline to Offshore Process Piping: High Performance Alloys. CRC Press. pp. 166–167. ISBN 978-0-8247-2481-8.
- ^ Thomas Kahl, Kai-Wilfrid Schröder, F. R. Lawrence, W. J. Marshall, Hartmut Höke, Rudolf Jäckh "Aniline" in Ullmann's Encyclopedia of Industrial Chemistry 2007; John Wiley & Sons: New York.doi:10.1002/14356007.a02_303
- ^ Béchamp, Antoine (1854). "De l'action des protosels de fer sur la nitronaphtaline et la nitrobenzine. nouvelle méthode de formation des bases organiques artificielles de Zinin". Annales de chimie et de physique. 42: 186–196.
- ^ Mahood, S. A.; Schaffner, P. V. L. (1931). "2,4-Diaminotoluene". Org. Synth. 11: 32. doi:10.15227/orgsyn.011.0032.
- ^ Bavin, G. David (1960). "2-Aminofluorene". Org. Synth. 40: 5. doi:10.15227/orgsyn.040.0005.
- ^ Mendenhall, P. M. G.; Smith, Peter A. S. (1966). "2-Nitrocarbazole". Org. Synth. 46: 85. doi:10.15227/orgsyn.046.0085.
- ^ M. J. Leonard; A. R. Lingham; J. O. Niere; N. R. C. Jackson; P. G. McKay; H. M. Hϋgel (6 Mar 2014). "Alternative synthesis of the anti-baldness compound RU58841". RSC Advances. 4 (27): 14143–14148. doi:10.1039/c4ra00332b.
- ^ Wang, Zerong (2010). "Béchamp Reduction". Comprehensive Organic Name Reactions and Reagents. doi:10.1002/9780470638859.conrr063. ISBN 9780470638859.
- Name reactions
- Organic redox reactions
- Chemical reaction stubs