Bladder cancer
| Bladder cancer | |
|---|---|
 | |
| Transitional cell carcinoma of the bladder. The white in the bladder is contrast. | |
| Specialty | Oncology, urology |
| Symptoms | Blood in the urine, pain with urination[1] |
| Usual onset | 65 to 84 years old[2] |
| Types | Transitional cell carcinoma, squamous cell carcinoma, adenocarcinoma[1] |
| Risk factors | Smoking, family history, prior radiation therapy, frequent bladder infections, certain chemicals[1] |
| Diagnostic method | Cystoscopy with tissue biopsies[1] |
| Treatment | Surgery, radiation therapy, chemotherapy, immunotherapy[1] |
| Prognosis | Five-year survival rates ~77% (US)[2] |
| Frequency | 549,000 new cases (2018)[3] |
| Deaths | 200,000 (2018)[3] |
Bladder cancer is any of several types of cancer arising from the tissues of the urinary bladder.[1] Symptoms include blood in the urine, pain with urination, and low back pain.[1] It is caused when epithelial cells that line the bladder become malignant. [4]
Risk factors for bladder cancer include smoking, family history, prior radiation therapy, frequent bladder infections, and exposure to certain chemicals.[1] The most common type is transitional cell carcinoma.[1] Other types include squamous cell carcinoma and adenocarcinoma.[1] Diagnosis is typically by cystoscopy with tissue biopsies.[5] Staging of the cancer is determined by transurethral resection and medical imaging.[1][6][7]
Treatment depends on the stage of the cancer.[1] It may include some combination of surgery, radiation therapy, chemotherapy, or immunotherapy.[1] Surgical options may include transurethral resection, partial or complete removal of the bladder, or urinary diversion.[1] The typical five-year survival rates in the United States is 77%, Canada is 75%, and Europe is 68%.[2][8][9]
Bladder cancer, as of 2018, affected about 1.6 million people globally with 549,000 new cases and 200,000 deaths.[3] Age of onset is most often between 65 and 84 years of age.[2] Males are more often affected than females.[2] In 2018, the highest rate of bladder cancer occurred in Southern and Western Europe followed by North America with rates of 15, 13, and 12 cases per 100,000 people.[3] The highest rates of bladder cancer deaths were seen in Northern Africa and Western Asia followed by Southern Europe.[3]
Signs and symptoms[]

Bladder cancer characteristically causes blood in the urine, which may be visible or detectable only by microscope. Blood in the urine is the most common symptom in bladder cancer, and is painless. Visible blood in the urine may be of only short duration, and a urine test may be required to confirm non-visible blood. Between 80 and 90% of people with bladder cancer initially presented with visible blood.[10] Blood in the urine may also be caused by other conditions, such as bladder or ureteric stones, infection, kidney disease, kidney cancers or vascular malformations, though these conditions (except kidney cancers) would typically be painful.[citation needed]
Other possible symptoms include pain during urination, frequent urination, or feeling the need to urinate without being able to do so. These signs and symptoms are not specific to bladder cancer, and may also be caused by non-cancerous conditions, including prostate infections, overactive bladder or cystitis. Some rare forms of bladder cancer like urachal adenocarcinoma produce mucin, which is then excreted in the urine causing it to be thick.[11]
People with advanced disease may have pelvic or bony pain, lower-extremity swelling, or flank pain.[12] Rarely, a palpable mass can be detected on physical examination.[13]
Causes[]
Tobacco smoking is the main known contributor to urinary bladder cancer; in most populations, smoking is associated with over half of bladder cancer cases in men and one-third of cases among women,[14] however these proportions have reduced over recent years since there are fewer smokers in Europe and North America.[15] There is an almost linear relationship between smoking duration (in years), pack years and bladder cancer risk. A risk plateau at smoking about 15 cigarettes a day can be observed (meaning that those who smoke 15 cigarettes a day are approximately at the same risk as those smoking 30 cigarettes a day). Smoking (cigar, pipe, Egyptian waterpipe and smokeless tobacco) in any form increases the risk for bladder cancer.[16] Quitting smoking reduces the risk. Risk of bladder cancer decreases by 30% within 1–4 years and continues to decrease by 60% at 25 years after smoking cessation.[17] However, former smokers will most likely always be at a higher risk of bladder cancer compared to people who have never smoked.[15] Passive smoking also appear to be a risk.[18][19]
Opium consumption increases the risk of bladder cancer by 3-fold and concurrent use of opium and smoking increases the risk of bladder cancer by 5 times compared to the general population.[20]
Thirty percent of bladder tumors probably result from occupational exposure in the workplace to carcinogens. Occupational or circumstantial exposure to the following substances has been implicated as a cause of bladder cancer; benzidine (dyes manufacturing), 4-aminobiphenyl (rubber industry), 2-naphtylamine (azo dyes manufacturing, foundry fumes, rubber industry, cigarette smoke and cancer research), phenacetin (analgesic), arsenic and chlorinated aliphatic hydrocarbons in drinking water, auramine (dye manufacturing), magenta (dye manufacturing), ortho-toluidine (dye manufacturing), epoxy and polyurethane resin hardening agents (plastics industry), chlornaphazine, coal-tar pitch.[21][22][23][24][25] Occupations at risk are bus drivers, rubber workers, painters, motor mechanics, leather (including shoe) workers, blacksmiths, machine setters, and mechanics.[26][27] Hairdressers are thought to be at risk as well because of their frequent exposure to permanent hair dyes.[28]
Infection with Schistosoma haematobium (bilharzia or schistosomiasis) may cause bladder cancer, specially of the squamous cell type.[29] Schistosoma eggs induces a chronic inflammatory state in the bladder wall resulting in tissue fibrosis.[30] Higher levels of N-nitroso compounds has been detected in urine samples of people with schistosomiasis.[31] N-Nitroso compounds have been implicated in the pathogenesis of schistosomiasis related bladder cancer. They cause alkylation DNA damage, specially Guanine to Adenine transition mutations in the HRAS and p53 tumor suppressor gene.[32] Mutations of p53 are detected in 73% of the tumors, BCL-2 mutations accounting for 32% and the combination of the two accounting for 13%.[33] Other causes of squamous cell carcinoma of the bladder include chronic catheterizations in people with a spinal cord injury and history of treatment with cyclophosphamide.[34][35]
Ingestion of aristolochic acid present in many Chinese herbal medications has been shown to cause urothelial carcinoma and kidney failure.[36] Aristolochic acid activates peroxidase in the urothelium and causes transversion mutation in the TP53 tumor suppressor gene.[citation needed]
People who undergo external beam radiotherapy (EBRT) for prostate cancer have a higher risk of developing invasive bladder cancer.[37]
In addition to these major risk factors there are also numerous other modifiable factors that are less strongly (i.e. 10–20% risk increase) associated with bladder cancer, for example, obesity.[38] Although these could be considered as minor effects, risk reduction in the general population could still be achieved by reducing the prevalence of a number of smaller risk factor together.[39]
Genetics[]
Mutations in FGFR3, TP53, PIK3CA, KDM6A, ARID1A, KMT2D, HRAS, TERT, KRAS, CREBBP, RB1 and TSC1 genes may be associated with some cases of bladder cancer.[40][41][42] Deletions of parts or whole of chromosome 9 is common in bladder cancer.[43] Low grade cancer are known to harbor mutations in RAS pathway and the fibroblast growth factor receptor 3 (FGFR3) gene, both of which play a role in the MAPK/ERK pathway. p53 and RB gene mutations are implicated in high-grade muscle invasive tumors.[44] Eighty nine percent of muscle invasive cancers have mutations in chromatin remodeling and histone modifying genes.[45] Deletion of both copies of the GSTM1 gene has a modest increase in risk of bladder cancer. GSTM1 gene product glutathione S-transferase M1 (GSTM1) participates in the detoxification process of carcinogens such as polycyclic aromatic hydrocarbons found in cigarette smoke.[46] Similarly, mutations in NAT2 (N-acetyltransferase) is associated with increased risk for bladder cancer. N-acetyltransferase helps in detoxification of carcinogens like aromatic amines (also present in cigarette smoke).[47] Various single-nucleotide polymorphisms in PSCA gene present on chromosome 8 have shown to increase the risk for bladder cancer. PSCA gene promoter region has an androgen response region. Loss of reactivity of this region to androgens is hypothesized as a cause of more number of aggressive tumors in women (unlike in men who have higher amount of androgen).[48]
Muscle invasive bladder cancer are heterogeneous in nature. In general, they can be genetically classified into basal and luminal subtypes. Basal subtype show alterations involving RB and NFE2L2 and luminal type show changes in FGFR3 and KDM6A genes.[49] Basal subtype are subdivided into basal and claudin low-type group and are aggressive and show metastasis at presentation, however they respond to platinum based chemotherapy. Luminal subtype can be subdivided into p53-like and luminal. p53-like tumors of luminal subtype although not as aggressive as basal type, show resistance to chemotherapy[50]
Diagnosis[]


Currently, the best diagnosis of the state of the bladder is by way of cystoscopy, which is a procedure in which a flexible or rigid tube (called a cystoscope) bearing a camera and various instruments is introduced into the bladder through the urethra. The flexible procedure allows for a visual inspection of the bladder, for minor remedial work to be undertaken and for samples of suspicious lesions to be taken for a biopsy. A rigid cystoscope is used under general anesthesia in the operating room and can support remedial work and biopsies as well as more extensive tumor removal. Unlike papillary lesion, which grow into the bladder cavity and are readily visible, carcinoma in situ lesion are flat and obscure. Detection of carcinoma in situ lesions requires multiple biopsies from different areas of interior bladder wall.[51] Photodynamic detection (blue light cystoscopy) can aid in the detection of carcinoma in situ. In photodynamic detection, a dye is instilled into the bladder with the help of a catheter. Cancer cells take up this dye and are visible under blue light, providing visual clues on areas to biopsied or resected.[52]
However, visual detection in any form listed above, is not sufficient for establishing pathological classification, cell type or the stage of the present tumor. A so-called cold cup biopsy during an ordinary cystoscopy (rigid or flexible) will not be sufficient for pathological staging either. Hence, a visual detection needs to be followed by transurethral surgery. The procedure is called transurethral resection of bladder tumor (TURBT). Further, a rectal and vaginal bimanual examination should be carried out before and after the TURBT to assess whether there is a palpable mass or if the tumour is fixed ("tethered") to the pelvic wall. The pathological classification and staging information obtained by the TURBT-procedure, is of fundamental importance for making the appropriate choice of ensuing treatment and/or follow-up routines.[53]
If invasive or high grade (includes carcinoma in situ) cancer is detected on TURBT, an MRI and/or CT scan of the abdomen and pelvis or urogram and CT chest or x-ray chest should be conducted for disease staging and to look for cancer spread (metastasis). Increase in alkaline phosphatase levels without evidence of liver disease should be evaluated for bone metastasis by a bone scan.[54] Although 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT has been explored as a viable method for staging, there is no consensus to support its role in routine clinical evaluations.[52]
Urine cytology can be obtained in voided urine or at the time of the cystoscopy ("bladder washing"). Cytology is not very sensitive for low-grade or grade 1 tumors (a negative result cannot reliably exclude bladder cancer) but has a high specificity (a positive result reliably detects bladder cancer).[55] There are newer non-invasive urine bound markers available as aids in the diagnosis of bladder cancer, including human complement factor H-related protein, high-molecular-weight carcinoembryonic antigen, and nuclear matrix protein 22 (NMP22).[56] In United States the FDA has approved NMP22, NMP22 BladderChek, and UroVysion tests for detection and surveillance of bladder cancer and ImmunoCyt, BTA-TRAK, and BTA-STAT tests have been approved for surveillance only. BTA-STAT and BladderChek can be performed in the clinic and others are done in the laboratory.[57][58] Other non-invasive urine based tests include the CertNDx Bladder Cancer Assay, which detects FGFR3 mutation and Urine Bladder Cancer test (UBC), which is a sandwich ELISA for Cytokeratin 8/18 fragment. Likewise, NMP22 is a sandwich ELISA and NMP22 BladderChek is a dipstick immunoassay, both of them detect nuclear mitotic apparatus protein (NuMA) tumor marker (a type of nuclear matrix protein).[59] UroVysion is a fluorescence in situ hybridization which detects aneuploidy in chromosomes 3, 7, 17 and loss of the 9p21 locus.[60][61] ImmunoCyt is an Immunofluorescence test which detects glycosylated CEA and MUCIN-like antigens (M344, LDQ10, 19A11).[59][60] BTA-STAT is a dipstick immunoassay for detection of human complement factor H-related protein. BTA-TRAK is a sandwich ELISA which also detects human complement factor H-related protein.[59] Sensitivities across biomarkers ranged from 0.57 to 0.82 and specificities from 0.74 to 0.88. Biomarkers fared better when used in combination with urine cytology than when used alone. However, detection accuracy is poor for low grade cancers and 10% cancers are still missed.[57] Current guidelines do not recommended using urinary biomarkers for detection and surveillance.[62]
Classification[]

| Type | Relative incidence | Subtypes |
|---|---|---|
| Transitional cell carcinoma | 95%[63][64] | Papillary (70%[63]) |
| Non-papillary (30%[63]) | ||
| Non-transitional cell carcinoma | 5% [63][64] | Squamous cell carcinomas, adenocarcinomas, sarcomas, small cell carcinomas, and secondary deposits from cancers elsewhere in the body.[64] |
Non-papillary carcinoma includes carcinoma in situ (CIS), microinvasive carcinoma and frankly invasive carcinoma.[65] Carcinoma in situ (CIS) invariably consists of cytologically high-grade tumour cells.[66]
Transitional cell carcinoma can undergo differentiation (25%) into its variants.[65][67][68] When seen under a microscope, papillary transitional cell carcinoma can present in its typical form or as one of its variations (squamous, glandular differentiation or micropapillary variant). Different variations of non-papillary transitional cell carcinoma are listed below.
| Variant | Histology | Percentage of non-papillary cases | Implications[69] |
|---|---|---|---|
| Squamous differentiation | Presence of intercellular bridges or keratinization | 60% | Outcomes similar to conventional transitional cell carcinoma |
| Glandular differentiation | Presence of true glandular spaces | 10% | |
| Sarcomatoid foci | Presence of both epithelial and mesenchymal differentiation | 7% | Clinically aggressive[70] |
| Micropapillary variant | Resembles papillary serous carcinoma of the ovary or resembling micropapillary carcinoma of breast or lung[71] | 3.7% | Clinically aggressive, early cystectomy recommended |
| Urothelial carcinoma with small tubules and microcystic form | Presence of cysts with a size range of microscopic to 1-2mm | Rare | |
| Lymphoepithelioma-like carcinoma | Resembles lymphoepithelioma of the nasopharynx | ||
| Lymphoma-like and plasmacytoid variants | Malignant cells resemble cells of malignant lymphoma or plasmacytoma | ||
| Nested variant | Histologically look similar to von Brunn's nests | Can be misdiagnosed as benign von Brunn's nests or non-invasive low-grade papillary urothelial carcinoma | |
| Urothelial carcinoma with giant cells | Presence of epithelial tumour giant cells and looks similar to giant cell carcinoma of the lung | ||
| Trophoblastic differentiation | Presence of syncytiotrophoblastic giant cells or choriocarcinomatous differentiation, may express HCG | ||
| Clear cell variant | Clear cell pattern with glycogen-rich cytoplasm | ||
| Plasmacytoid | Cells with abundant lipid content, mimic signet ring cell adenocarcinoma of stomach/ lobular breast cancer | Clinically aggressive, propensity for peritoneal spread | |
| Unusual stromal reactions | Presence of following; pseudosarcomatous stroma, stromal osseous or cartilaginous metaplasia, osteoclast-type giant cells, lymphoid infiltrate |
Staging[]
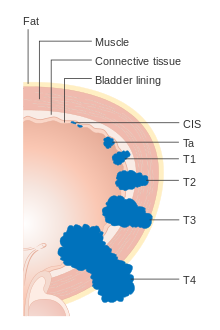




Bladder cancer is staged (classified by the extent of spread of the cancer) and graded (how abnormal and aggressive the cells appear under the microscope) to determine treatments and estimate outcomes. Staging usually follows the first transurethral resection of bladder tumor (TURBT). Papillary tumors confined to the mucosa or which invade the lamina propria are classified as Ta or T1. Flat lesion are classified as Tis. Both are grouped together as non-muscle invasive disease for therapeutic purposes.[citation needed]
In the TNM staging system (8th Edn. 2017) for bladder cancer:[72][73]
T (Primary tumour)
- TX Primary tumour cannot be assessed
- T0 No evidence of primary tumour
- Ta Non-invasive papillary carcinoma
- Tis Carcinoma in situ ('flat tumour')
- T1 Tumour invades subepithelial connective tissue
- T2a Tumour invades superficial muscle (inner half of the detrusor muscle)[74]
- T2b Tumour invades deep muscle (outer half of the detrusor muscle)[74]
- T3 Tumour invades perivesical tissue:
- T3a Microscopically
- T3b Macroscopically (extravesical mass)
- T4a Tumour invades prostate, uterus or vagina
- T4b Tumour invades pelvic wall or abdominal wall
N (Lymph nodes)
- NX Regional lymph nodes cannot be assessed
- N0 No regional lymph node metastasis
- N1 Metastasis in a single lymph node in true pelvis (hypogastric, obturator, external iliac, or presacral nodes)
- N2 Metastasis in multiple lymph nodes in true pelvis (hypogastric, obturator, external iliac, or presacral nodes)
- N3 Metastasis in common iliac lymph nodes
M (Distant metastasis)
- MX Distant metastasis cannot be assessed
- M0 No distant metastasis
- M1 Distant metastasis.
- M1a: The cancer has spread only to lymph nodes outside of the pelvis.
- M1b: The cancer has spread other parts of the body.
The most common sites for bladder cancer metastases are the lymph nodes, bones, lung, liver, and peritoneum.[75] The most common sentinel lymph nodes draining bladder cancer are obturator and internal iliac lymph nodes. The location of lymphatic spread depends on the location of the tumors. Tumors on the superolateral bladder wall spread to external iliac lymph nodes. Tumors on the neck, anterior wall and fundus spread commonly to the internal iliac lymph nodes.[76] From the regional lymph nodes (i.e. obturator, internal and external lymph nodes) the cancer spreads to distant sites like the common iliac lymph nodes and paraaortic lymph nodes.[77] Skipped lymph node lesions are not seen in bladder cancer.[76]
Numerical
The stages above can be integrated into a numerical staging (with Roman numerals) as follows:[78]
| Stage | Tumor | Nodes | Metastasis | 5-year survival in the US[79] |
|---|---|---|---|---|
| Stage 0a | Ta | N0 | M0 | 98% |
| Stage 0is | Tis | N0 | M0 | 95% |
| Stage I | T1 | N0 | M0 | 63% |
| Stage II | T2a | N0 | M0 | |
| T2b | ||||
| Stage IIIA | T3a | N0 | M0 | 35% |
| T3b | ||||
| T4a | ||||
| T1-4a | N1 | |||
| Stage IIIB | T1-4a | N2 | M0 | |
| N3 | ||||
| Stage IVA | T4b | Any N | M0 | |
| Any T | M1a | |||
| Stage IVB | Any T | ny N | M1b | 5% |
Grading[]
According to WHO classification (1973) bladder cancers are histologically graded into:[80]
- G1 – Well differentiated,
- G2 – Moderately differentiated
- G3 – Poorly differentiated
WHO classification (2004/2016)[81][82]
- Papillary lesions
- Urothelial Papilloma
- Papillary urothelial neoplasm of low malignant potential (PUNLMP)
- Low Grade
- High Grade
- Flat lesions
- Urothelial proliferation of uncertain malignant potential
- Reactive atypia
- Atypia of unknown significance
- Urothelial dysplasia
- Urothelial CIS (always high grade)
- Primary
- Secondary
- Concurrent
Risk stratification[]
People with non-muscle invasive bladder cancer (NMIBC), are risk-stratified based on clinical and pathological factors so that they are treated appropriately depending on their probability of having progression and/or recurrence.[83] People with non-muscle invasive tumors are categorized into low-risk, intermediate-risk and high-risk or provided with a numerical risk score. Risk-stratification framework is provided by American Urology Association/Society of Urological Oncology (AUA/SUO stratification), European Association of Urology (EAU) guidelines, European Organization for Research and Treatment of Cancer (EORTC) risk tables and Club Urológico Español de Tratamiento Oncológico (CUETO) scoring model.[84][85][86]
| Low risk | Intermediate risk | High risk |
|---|---|---|
| Low grade solitary Ta tumor, smaller than 3 cm | Recurrence within 1 year, Low grade Ta tumor | High grade T1 |
| Papillary urothelial neoplasm of low malignant potential | Solitary low grade Ta tumor, bigger than 3 cm | Any recurrent tumor or any high grade Ta |
| Low grade Ta, multifocal tumors | High grade Ta, bigger than 3 cm (or multifocal) | |
| High grade Ta, smaller than 3 cm | Any carcinoma in situ | |
| Low grade T1 | Any BCG failure in high grade tumors | |
| Any variant histology | ||
| Any lymphovascular invasion | ||
| Any high grade prostatic urethral involvement |
The EORTC and CUETO model use a cumulative score obtained from individual prognostic factors, which are then converted into risk of progression and recurrence. The six prognostic factors included in the EORTC model are number of tumors, recurrence rate, T-stage, presence of carcinoma-in-situ and grade of the tumor. Scoring for recurrence in the CUETO model incorporates 6 variables; age, gender, grade, tumor status, number of tumors and presence of tis. For progression scoring the previous 6 variables plus T stage is used.[87][88]
| Model | Cumulative score for recurrence | Recurrence at 1-year (%) | Recurrence at 5-year (%) |
|---|---|---|---|
| EORTC | 0 | 15 | 31 |
| 1-4 | 24 | 46 | |
| 5-9 | 38 | 62 | |
| 10-17 | 61 | 78 | |
| CUETO | 0-4 | 8.2 | 21 |
| 5-6 | 12 | 36 | |
| 7-9 | 25 | 48 | |
| 10-16 | 42 | 68 |
| Model | Cumulative score for progression | Progression at 1-year (%) | Progression at 5-year (%) |
|---|---|---|---|
| EORTC | 0 | 0.2 | 0.8 |
| 2-6 | 1 | 6 | |
| 7-13 | 5 | 17 | |
| 12-23 | 17 | 45 | |
| CUETO | 0-4 | 1.2 | 3.7 |
| 5-6 | 3 | 12 | |
| 7-9 | 5.5 | 21 | |
| 10-16 | 14 | 34 |
Prevention[]
As of 2019, there is limited high level evidence to suggest that eating vegetable and fruits decreases the risk of bladder cancer.[47] A 2008 study concluded that "specific fruit and vegetables may act to reduce the risk of bladder cancer."[91] Fruit and yellow-orange vegetables, particularly carrots and those containing selenium,[92] are probably associated with a moderately reduced risk of bladder cancer. Citrus fruits and cruciferous vegetables were also identified as having a possibly protective effect. However an analysis of 47,909 men in the Health Professionals Follow-Up Study showed little relation between cancer reduction and high consumption of fruits and vegetables overall, or yellow or green leafy vegetables specifically, compared to the reduction seen among those men who consumed large amounts of cruciferous vegetables. An inverse relation between in-takes of flavonols and lignans (diphenolic compounds found in whole grains, legumes, fruits and vegetables) and aggressive bladder cancer has also been described.[93]
While it is suggested that the polyphenol compounds in tea may have an inhibitory effect on bladder tumor formation and growth, there is limited evidence to suggesting drinking tea decreases bladder cancer risk.[47]
In a 10-year study involving almost 49,000 men, researchers found that men who drank at least 1.44 L of water (around 6 cups) per day had a reduced risk of bladder cancer when compared with men who drank less. It was also found that: "the risk of bladder cancer decreased by 7% for every 240 mL of fluid added".[94] The authors proposed that bladder cancer might partly be caused by the bladder directly contacting carcinogens that are excreted in urine, although this has not yet been confirmed in other studies.[91]
Screening[]
As of 2019 there is insufficient evidence to determine if screening for bladder cancer in people without symptoms is effective or not.[95]
Treatment[]

The treatment of bladder cancer depends on how deeply the tumor invades into the bladder wall.
Treatment strategies for bladder cancer include:[96][97]
- Non-muscle invasive: transurethral resection of bladder tumor (TURBT) with or without intravesical chemotherapy or immunotherapy
- Muscle invasive
- Stage II/Stage IIIA: radical cystectomy plus neoadjuvant chemotherapy (multimodal therapy, preferred) or transurethral resection with chemoradiation (trimodal therapy, highly selected people) or partial cystectomy plus neoadjuvant chemotherapy (in highly selected people)
- Stage IIIB/IVA: cisplatin-based chemotherapy followed by radical cystectomy or chemoradiation or observation depending on treatment response
- Stage IVB (locally advanced; unresectable tumors): palliative radiotherapy
- Metastatic disease: cisplatin-based chemotherapy
- Metastatic disease but unfit for cisplatin-based chemotherapy: carboplatin-based chemotherapy
- Metastatic disease with contraindication for chemotherapy: checkpoint inhibitors if programmed death ligand 1 (PD L1) positive
- Squamous cell carcinoma or adenocarcinoma of bladder: radical cystectomy
Non-muscle invasive[]
Transurethral resection[]
Non-muscle invasive bladder cancer (those not entering the muscle layer of the bladder) can be "shaved off" using an electrocautery device attached to a cystoscope, which in that case is called a resectoscope. The procedure is called transurethral resection of bladder tumor (TURBT) and serves primarily for pathological staging. In case of non-muscle invasive bladder cancer the TURBT is in itself the treatment, but in case of muscle invasive cancer, the procedure is insufficient for final treatment.[53] Additionally, blue light cystoscopy with optical-imaging agent Hexaminolevulinate (HAL) is recommended at initial TURBT to increase lesion detection (especially carcinoma in situ) and improve resection quality thereby reducing recurrence.[98][99] It is important to assess the quality of the resection, if there is evidence of incomplete resection or there is no muscle in the specimen (without which muscle invasiveness cannot be determined) a second TURBT is strongly recommended. Moreover, nearly half of the people with high grade non-invasive disease have residual tumor after primary TURBT, in such cases a second TURBT is important for avoiding under-staging.[100][101] At this point classifying people into risk groups is recommended. Treatment and surveillance for different risk groups is indicated in the table below.[citation needed]
Another method for reducing recurrence of tumors is medication after TURBT surgery. The two most common medicines used for this purpose are Bacillus Calmette–Guérin (BCG) and mitomycin.[102] For people who have already had a TURBT procedure, BCG may lead to similar risk of death and may reduce the risk of tumor recurrence.[102] However, this medication may increase the risk of serious unwanted side effects.[102] More research is needed to confirm these results.
Chemotherapy[]

A single instillation of chemotherapy into the bladder after primary TURBT has shown benefit in deceasing recurrence by 35% in non-muscle invasive disease.[103] Medications which can used for this purpose are mitomycin C (MMC), epirubicin, pirarubicin and gemcitabine. Instillation of post-operative chemotherapy should be conducted within first few hours after TURBT. As time progress residual tumor cells are known to adhere firmly and are covered by extracellular matrix which decrease the efficacy of the instillation.[101] The most common side effect is chemical cystitis and skin irritation.[103] If there is a suspicion of bladder perforation during TURBT, chemotherapy should not be instilled into the bladder as serious adverse events are known to occur due to drug extravasation. Studies have shown that efficacy of chemotherapy is increased by the use of Device assisted chemotherapy .[104] These technologies use different mechanisms to facilitate the absorption and action of a chemotherapy drug instilled directly into the bladder. Another technology – electromotive drug administration (EMDA) – uses an electric current to enhance drug absorption after surgical removal of the tumor.[105][106] Another technology, thermotherapy, uses radio-frequency energy to directly heat the bladder wall, which together with chemotherapy (chemohyperthermia) shows a synergistic effect, enhancing each other's capacity to kill tumor cells.[107]
Immunotherapy[]
Immunotherapy by Bacillus Calmette–Guérin (BCG) delivery into the bladder is also used to treat and prevent the recurrence of NMIBC.[108] BCG is a vaccine against tuberculosis that is prepared from attenuated (weakened) live bovine tuberculosis bacillus, Mycobacterium bovis, that has lost its virulence in humans. BCG immunotherapy is effective in up to 2/3 of the cases at this stage, and in randomized trials has been shown to be superior to standard chemotherapy.[109] The exact mechanism by which BCG prevents recurrence is unknown. However, it has been shown that the bacteria are taken up by the cancer cells.[110] The infection of these cells in the bladder may trigger a localized immune reaction which clears residual cancer cells.[111][112]
BCG is delivered as induction and a maintenance course. The induction course consists of 6-week course of intravesical and percutaneous BCG.[113] This is followed by a maintenance course. There is no consensus regarding the maintenance schedule, however the most commonly followed is the Southwestern Oncology Group (SWOG) schedule.[114] The SWOG maintenance schedule consists of intravesical and percutaneous BCG every week for 3 weeks given at 3, 6, 12, 18, 24, 30 and 36 months.[113] Three weekly maintenance regimen with induction has shown complete response rate of 84% compared to 69% in people who received 6-week induction BCG only at 6 months. Many studies have explored alternate treatment schedules and regimes of BCG but has shown no clinical significance.[113] Efficacy of different strains of BCG (Connaught, TICE, Pasteur, Tokio-172) has been shown not to be different however, there is no high-level evidence.[115]
Side effects of BCG therapy include cystitis, prostatitis, epididymo-orchitis, balanitis, ureteral obstruction, bladder contraction, mycobacterial osteomyelitis, reactive arthritis, mycobacterial pneumonia, granulomatous hepatitis, granulomatous nephritis, interstitial nephritis, infectious vasculitis and disseminated infection.[116][117]
Local infection (i.e. prostatitis, epididymo-orchitis, balanitis) because of BCG should be treated with triple tubercular therapy, with one of the drug being fluoroquinolone for 3 to 6 months. In people with systemic infections, BCG therapy should be stopped and anti-tubercular multidrug treatment for at-least 6 months should be started. Medications that can be used for this treatment are INH, rifampicin, ethambutol, fluoroquinolones, clarithromycin, aminoglycosides, and doxycycline. BCG strains are not sensitive to pyrazinamide therefore, it should not be a part of anti-tubercular treatment.[118]
BCG treatment failure[]
BCG treatment failure can be classified into 3 groups; BCG relapse, BCG-refractory and BCG-intolerant. In BCG relapse, tumor reoccurs after a disease free period. BCG-refractory tumors are the ones which do not respond to induction and maintenance doses of BCG or which progress during therapy. In BCG-intolerant, tumor reoccurs due to incomplete treatment as the person receiving it is unable to tolerate an induction course of BCG. Around 50% of the people fail BCG treatment and would require further treatment.[113]
People whose tumors recurred after treatment with BCG or who were unresponsive to treatment, are more difficult to treat.[119] In such people a radical cystectomy is recommendation[120][121] In people who do not show response to BCG therapy and are unfit or unwilling to undergo radical cystectomy, salvage therapies can be considered. Salvage therapy include intravesical chemotherapy with agents such as valrubicin, gemcitabine or docetaxel, chemoradiation or chemohyperthermia.[122]
| Risk | Other considerations | Chemotherapy | Immunotherapy (BCG) | Cystoscopy (surveillance) | Imaging (surveillance) |
|---|---|---|---|---|---|
| Low | at 3-months followed by cystoscopy at 12-months, then yearly for 5-years | CT/MR urography and CT/MRI of abdomen and pelvis at baseline | |||
| Intermediate | Primary tumor with history of chemotherapy | Intravesical chemotherapy for 1 year OR Intravesical BCG for 1 year (preferred) | at 3-months with cytology followed by once every 3–6 months for 5-years and then yearly | CT/MR urography and CT/MRI of abdomen and pelvis at baseline | |
| Recurrent tumors with history of previous chemotherapy | Intravesical BCG for 1 year | ||||
| High | Intravesical BCG for 3 year (as tolerated) | at 3-months with cytology followed by once every 3-months for 2-years after that, 6 monthly for 5 years then yearly | CT/MR urography and CT/MRI of abdomen and pelvis at baseline, CT/MR urography 1-2 yearly for 10 years | ||
| T1G3/High grade, Lymphovascular invasion, Presence of variant histology | Consider radical cystectomy | ||||
Muscle invasive[]

Multimodal therapy (standard treatment)[]
Untreated, non-muscle invasive tumors may gradually begin to infiltrate the muscular wall of the bladder (muscle invasive bladder cancer). Tumors that infiltrate the bladder wall require more radical surgery, where part (partial cystectomy) or all (radical cystectomy) of the bladder is removed (a cystectomy) and the urinary stream is diverted into an isolated bowel loop (called an ileal conduit or urostomy). In some cases, skilled surgeons can create a substitute bladder (a neobladder) from a segment of intestinal tissue, but this largely depends upon a person's preference, age of the person, renal function, and the site of the disease.[citation needed]
A bilateral pelvic lymphadenectomy should accompany radical cystectomy. At minimum, a standard template of lymphadenectomy should be followed by removing the external and internal iliac and obturator lymph node.[123] When performing a lymphadenectomy, the surgeon can either remove lymph nodes from a smaller (standard) or from larger (extended) area. In comparison with a standard lymph node dissection, having an extended dissection may reduce a person’s likelihood of death from any reason, including dying from bladder cancer.[124] The extended procedure may lead to more serious unwanted effects and may or may not influence the likelihood of the cancer recurring over time.[124] The rate of not-so-serious side effects may be similar for both surgeries.[124]
Radical cystectomy has a significant morbidity associated with it. About 50-65% of the people experience complication within 90 days of surgery.[125][126] Mortality rates was 7% within 90 days of surgery. High volume centers have better outcomes than low volume centers.[127] Some centers are implementing Enhanced Recovery After Surgery (ERAS) society recommendations to decrease morbidity after radical cystectomy. However, due to lack of specific evidence in urologic surgery, ERAS protocol has not been widely incorporated in urologic clinical care.[128]
Even after surgical removal of bladder, 50% of the people with muscle invasive disease (T2-T4) develop metastatic disease within two years due to micrometastasis,.[129] In such, neoadjuvant chemotherapy (chemotherapy before main treatment, i.e. surgery) has shown to increase overall survival at 5 years from 45% to 50% with an absolute survival benefit of 5%.[130][131][132] Currently the two most used chemotherapy regimens for neoadjuvant chemotherapy are platinum based; methotrexate, vinblastine, doxorubicin, cisplatin (MVAC) and gemcitabine with cisplatin (GC).[133] Other regimens include dose dense MVAC (DDMVC) and cisplatin, methotrexate and vinblastine (CMV). Although, the optimal regimen has not been established, the preferred regimen for neoadjuvant therapy is MVAC.[133]
Role of adjuvant chemotherapy (chemotherapy after main treatment) is limited to people with high grade tumours (pT3/T4and/or N+) and who have not been treated with neoadjuvant therapy.[123] Adjuvant radiation therapy has not shown any advantage in bladder cancer treatment.[134]
| MVAC | DDMVAC | Gemcitabine + cisplatin |
|---|---|---|
| Methotrexate (30 mg/m2 IV) - day 1,15,22
Vinblastine (3 mg/m2 IV) - day 2, 15, 22 Doxorubicin (30 mg/m2 IV) - day 2 Cisplatin (70 mg/m2 IV) - day 2 Repeat every 4 weeks for 3 cycles |
Methotrexate (30 mg/m2 IV) - day 1 Vinblastine (3 mg/m2 IV) - day 2 Doxorubicin (30 mg/m2 IV) - day 2 Cisplatin (70 mg/m2 IV) - day 2 Granulocyte colony-stimulating factor (G-CSF) (240μg/m2 SC) - day 4-10 Repeat every 2 weeks for 3–4 cycles |
Gemcitabine (1,000 mg/m2 IV) - day 1,8,15 Cisplatin (70 mg/m2) - day 2 Repeat every 4 weeks for 4 cycles |
Trimodal therapy (alternative treatment)[]
A combination of radiation and chemotherapy (chemoradiation) in conjunction with transurethral (endoscopic) bladder tumor resection can be used as an alternative in certain people.[137] Review of available large data series on this so-called trimodality therapy has indicated similar long-term cancer specific survival rates, with improved overall quality of life as for people undergoing radical cystectomy with urinary reconstruction. However, currently no randomized control trials are available which has compared trimodal therapy with radical cystectomy. People who undergo trimodal therapy are usually highly selected and generally have T2 disease without hydronephrosis and/or carcinoma in-situ.[138] Five year cancer specific survival and overall survival after trimodal therapy is between 50% to 82% and 36% to 74%.[137]
In trimodal therapy, a maximal TURBT is conducted followed by chemoradiation therapy. Radiation sensitizing chemotherapy regimens consisting of cisplatin or 5-flurouracil and mitomycin C are used. Radiation therapy is via external bean radiotherapy (EBRT) with a target curative dose of 64-66 Gy.[139] Surveillance for progression or recurrence should conducted with the aid of CT scans, cystoscopies and urine cytology.[123] Side effects of chemoradiation include nausea, vomiting, loss of appetite, hair loss, mouth sores, diarrhea, constipation, increased risk of infections and bleeding and fatigue.[140]
In people who fail trimodal therapy, radical cystectomy is considered if there is muscle invasive or recurrent tumors. Around 25-30% fail treatment and undergo salvage radical cystectomy.[137] TURBT with intravesical therapy is indicated after treatment failure for non-muscle invasive disease.[123]
Partial cystectomy[]
In people with solitary tumor without concurrent carcinoma in situ in an area where a clean surgical margins can be achieved, a partial cystectomy with lymphadenectomy can be considered. Management plan including partial cystectomy should be accompanied with neoadjuvant chemotherapy.[97] In people with urachal adenocarcinoma of the bladder, a partial cystectomy with en-bloc resection of urachal ligament and umbilicus can be considered.[11]
Metastatic disease[]
First line treatment[]
Cisplatin-containing combination chemotherapy is the standard of care for metastatic bladder care.[141] Fitness for receiving cisplatin based chemotherapy is assessed before treatment. A person is deemed unfit if anyone of the following is true.[142]
- Eastern Cooperative Oncology Group performance status of 2
- Creatinine clearance < 60 mL/min
- Grade ≥ 2 hearing loss
- Grade ≥ 2 neuropathy
- New York Heart Association Class III heart failure
People who are deemed fit receive platinum based regimens; methotrexate, vinblastine, doxorubicin, with cisplatin (MVAC) or gemcitabine with cisplatin (GC). Alternative regimens include paclitaxel with gemcitabine and cisplatin (PCG, triple therapy) and cisplatin, methotrexate and vinblastine (CMV). Response rate for cisplatin-based combination ranges from 39 to 65% and complete response is seen in 12-35% of the people.[143] MVAC is better tolerated if it is combined with granulocyte colony-stimulating factor and the regimen is known as dose dense MVAC regimen (DDMVAC). This combination has shown to decease all cause mortality.[144] MVAC regimen is aggressive. Febrile neutropenia (fever due to decrease in white blood cells) occurs in 10 to 14% and death due to toxicity in about 3-4%. Common side effects of MVAC include suppression of bone marrow, fever due to decrease in white blood cells, sepsis, mucositis, and nausea and vomiting.[143] In contrast, the GC regimen has shown lower rates of neutropenic sepsis and grade 3/4 mucositis compared to MVAC.[145] Efforts have been made to increase tolerance of cisplatin-based regimen by replacing it with carboplatin-based chemotherapy. However, cisplatin-based therapy is superior to carboplatin-based chemotherapy in achieving overall and complete response.[146] Nevertheless, nearly half of the people with metastatic disease are "unfit" for cisplatin-based therapy. In such persons a combination of carboplatin and gemcitabine (GemCarbo) can be used as first line chemotherapy.[147] In people who are not eligible for any platinum based chemotherapy and have PD-L1 expression, Atezolizumab and Pembrolizumab can be used.[citation needed]
People with bone metastasis should receive bisphosphonates or denosumab to prevent skeletal related events (e.g. fractures, spinal cord compression, bone pain).[148]
| DDMVAC | Gemcitabine + Cisplatin |
|---|---|
| Methotrexate (30 mg/m2 IV) - day 1
Vinblastine (3 mg/m2 IV) - day 2 Doxorubicin (30 mg/m2 IV) - day 2 Cisplatin (70 mg/m2 IV) - day 2 Granulocyte colony-stimulating factor (G-CSF) (240μg/m2 SC) - day 4-10 Repeat every 2 weeks for 3–4 cycles |
Gemcitabine (1,000 mg/m2 IV) - day 1,8,15 Cisplatin (70 mg/m2) - day 2 Repeat every 4 weeks for 4 cycles |
| Atezolizumab (in PD-L1+) | Gemcitabine + Carboplatin | Pembrolizumab (in PD-L1+) |
|---|---|---|
| Atezolizumab (Atezolizumab 1200 mg IV)
every 3 weeks |
Gemcitabine (1,000 mg/m2 IV) - day 1,8 Carboplatin (4.5 × [glomerular filtration rate + 25]) - day 1 and every 3 weeks |
Pembrolizumab 200 mg every 3 weeks |
Second line treatment[]
Bladder cancer that is refractory or shows progression after platinum-based chemotherapy can be treated with second-line chemotherapy or immunotherapy.
The most commonly used second-line chemotherapy is single-agent regimes of Taxanes (Paclitaxel, nab-paclitaxel and Docetaxel). Other single-agent regimes include Vinflunine, a third generation vinca alkaloid (approved in Europe), Gemcitabine, Pemetrexed, Oxaliplatin, and Ifosfamide.[149][150][151] Side effects of Vinflunine include neutropenia, constipation, fatigue and anemia and has limited its use as a second line agent. Response to second-line chemotherapy occurs in 5%–20% people. Median progression free survival with second-line chemotherapy is 3–4 months.[152]
In people with fibroblast growth factor receptors (FGFR) mutations and fail standard platinum based chemotherapy erdafitinib can be used. Erdafitinib has shown a response rate of 40% in these patients.[153]
Five immunotherapy agents has been approved in the US for use in metastatic bladder cancer. They act by inhibiting programmed cell-death protein 1 (PD-1) or programmed cell-death ligand 1 (PD-L1). Pembrolizumab and nivolumab, and are inhibitors of programmed cell-death ligand 1 (PD-1). Avelumab, atezolizumab and durvalumab are inhibitors of PD-L1.[154][155]
Pembrolizumab probably improves overall survival a little and may slightly improve quality of life for people with urothelial cancer that has worsened after initial treatment when compared with continued chemotherapy.[156] However, pembrolizumab may have only minimal effects on the rate of death resulting from treatment or the rate at which the cancer advances.[156] Pembrolizumab may cause less serious side effects than chemotherapy.[156]
| Atezolizumab | Nivolumab | Pembrolizumab | Durvalumab | Avelumab |
|---|---|---|---|---|
| Atezolizumab 1200 mg IV
every 3 weeks |
Nivolumab 3 mg/kg IV every 2 weeks |
Pembrolizumab 200 mg every 3 weeks |
Durvalumab 10 mg/kg every 2 weeks for 12 months |
Avelumab 10 mg/kg IV every 2 weeks |
Surveillance and response[]
Contrast enhanced CT is used to monitor lung, liver, and lymph node metastases. A bone scan is used to detect and monitor bone metastasis.[157] Treatment response is measured using the Response evaluation criteria in solid tumors (RECIST) into one of the following groups; response (complete or partial), stable disease and progressive disease.[158]
Prognosis[]
People with non-muscle invasive tumors have a favorable outcome (5-year survival is 95% vs. 69% of muscle invasive bladder cancer).[159][160] However, 70% of them will have a recurrence after initial treatment with 30% of them presenting with muscle invasive disease.[161] Recurrence and progression to a higher disease stage have a less favorable outcome.[162]
Survival after radical cystectomy and pelvic lymph node dissection is dependent on the pathological stage. If the disease has not spread to the lymph node and is limited to the bladder (T1 or T2, N0) the 5-year survival is 78%. If it has spread locally around the region of the bladder with no lymph node involved (T3, N0) then the 5-year survival drops to 47%. In disease with lymph node spread (N+, irrespective of T stage) the 5-year survival is 31%. Locally advanced and metastatic disease drastically decreases survival, with a median survival of 3–6 months without chemotherapy. Cisplatin-based chemotherapy has increased the median survival to 15-months. However, the 5-year survival is still 15%.[163]
There are several prognostic factors which determine cancer specific survival after radical cystectomy. Factor with detrimental effect of cancer specific survival are old age, higher tumor grade and pathological stage, lymph node metastasis, presence of lymphovascular invasion and positive soft tissue margin.[164] Lymph node density (positive lymph nodes/total lymph nodes observed in the specimen from surgery) is a predictor of survival in lymph node positive disease. Higher the density lower is the survival.[165]
Quality of life[]
After radical cystectomy, urinary and sexual function remain inferior to the general population. People who have a neobladder have better emotional function and body image compared with ones with cutaneous diversion (who need to wear a bag to collect urine over their abdomen).[166] Social factors such family, relationships, health and finances contribute significantly for determining good quality of life in people who have been diagnosed with bladder cancer.[167]
A high percentage of people with bladder cancer have anxiety and depression.[168] People who are young, single and have advanced clinical disease have a high risk for getting diagnosed with a psychiatric illness post-treatment. People who suffer from psychiatric illness post treatment seem to have worse cancer specific and overall survival.[169][170]
Epidemiology[]
| Rank | Country | Overall | Men | Women |
|---|---|---|---|---|
| 1 | Lebanon | 25 | 40 | 9 |
| 2 | Greece | 21 | 40 | 4 |
| 3 | Denmark | 18 | 29 | 8 |
| 4 | Hungary | 17 | 27 | 9 |
| 5 | Albania | 16 | 27 | 6 |
| 5 | Netherlands | 16 | 26 | 8 |
| 7 | Belgium | 16 | 27 | 6 |
| 8 | Italy | 15 | 27 | 6 |
| 9 | Germany | 15 | 26 | 6 |
| 10 | Spain | 15 | 27 | 6 |

Globally, in 2017, bladder cancer resulted in 196,000 deaths, a 5.4% (age adjusted) decrease from 2007.[174] In 2018, the age adjusted rates of new cases of bladder cancer was 6 cases per 100,000 people and age adjusted death rate was 2 deaths per 100,000 people. Lebanon and Greece have the highest rate of new cases. In Lebanon, this high risk is attributed to high number of smokers and petrochemical air pollution.[175]
The risk of bladder cancer occurrence is four times higher in men than in women.[3] Smoking can only partially explain this higher rates in men in western hemisphere.[176] One other reason is that the androgen receptor, which is much more active in men than in women, may play a part in the development of the cancer.[177] This hypothesis is also supported by the fact that men undergoing androgen suppression therapy for unrelated reason seem to have a lower risk of developing bladder cancer.[178] In Africa, men are more prone to do field work and are exposed to infection with Schistosoma, this may explain to a certain extent the gap in incidence of squamous cell cancers in areas where bladder cancer is endemic.[176] However, women present with more aggressive disease and have worse outcomes than men. This difference in outcomes is attributed to numerous factors such as, difference in carcinogen exposure, genetics, social and quality of care.[48] One of the common signs of bladder cancer is hematuria and is quite often misdiagnosed as urinary tract infection in women, leading to a delay in diagnosis.[48] Moreover, as mentioned earlier PSCA gene may play a role in aggressive tumors in women.[citation needed]
Canada[]
Bladder cancer is the sixth most common cancer accounting for 3.7% of the new cancer cases. in 2018, 30,700 Canadians were living with bladder cancer, 9160 new cases were diagnosed and 2467 died from it.[179] In 2019, it is estimated that 11,800 new cases will be diagnosed and 2500 will die from it.[180] Among the 11,800 new cases, 9100 will be in men and 2700 in women. Of the 2500 who would die from it, 1800 will be men and 700 will be women.[180]
China[]
Bladder cancer is the 14th most common cancer and 16th most common cause of cancer death. In, 2018 it accounted for 82,300 new cases and 38,200 deaths.[181] The number of new cases is comparatively lower compared to its western counterparts. Majority of the people are diagnosed with non-muscle invasive disease (75%) and the rest have muscle invasive disease (25%). Carcinoma in situ was present in only 2.4% of the cases.[182]
Europe[]
In 2015, 131,000 news cases were diagnosed in the European Union with 40,000 deaths. It is the 5th most common cancer and 9th most common cause of cancer deaths.. The 5-year relative survival for bladder cancers diagnosed between 2000 and 2007 is 69%. Geographic variation is seen in survival rates with 5-year survival rates of 75% in Northern to 65% in Eastern Europe.[183]
United Kingdom[]
Bladder cancer is the ninth most common cancer in the UK accounting for 2.7% of all the new cancers cases. In 2018, there was 12,200 new cases and 6100 people died from it.[184]
United States[]
In the United States in 2019 80,470 cases and 17,670 deaths are expected making it the sixth most common type of cancer in the region.[2] Bladder cancer is the fourth most common type of cancer in men and the 12th most common cancer in women.[185] Around 62,000 men and 19,000 women are diagnosed with bladder cancer in 2019.[186] Between 2012 and 2016 annual rate of new bladder cancer cases decreased by one percent per year.[187]
References[]
- ^ Jump up to: a b c d e f g h i j k l m n "Bladder Cancer Treatment (PDQ®)–Patient Version - National Cancer Institute". www.cancer.gov. 11 May 2020. Retrieved 4 June 2020.
- ^ Jump up to: a b c d e f "Cancer of the Urinary Bladder - Cancer Stat Facts". SEER. Retrieved 30 October 2019.
- ^ Jump up to: a b c d e f "Bladder Cancer Factsheet" (PDF). Global Cancer Observatory. Retrieved 8 November 2019.
- ^ Heyes S.M., Prior K.N., Whitehead D., Bond M.J. Toward an Understanding of Patients' and Their Partners' Experiences of Bladder Cancer. Cancer Nurs.. 2020;43(5):E254-E263. doi:10.1097/NCC.0000000000000718
- ^ "Bladder Cancer Treatment". National Cancer Institute. 5 June 2017. Archived from the original on 14 July 2017. Retrieved 18 July 2017.
- ^ "EAU Guidelines: Non-muscle-invasive Bladder Cancer". Uroweb.
- ^ "Bladder Cancer - Stages and Grades". Cancer.Net. 25 June 2012.
- ^ "Bladder cancer". World Cancer Research Fund. 24 April 2018.
- ^ "Survival statistics for bladder cancer - Canadian Cancer Society". www.cancer.ca.
- ^ Avellino GJ, Bose S, Wang DS (June 2016). "Diagnosis and Management of Hematuria". The Surgical Clinics of North America. 96 (3): 503–15. doi:10.1016/j.suc.2016.02.007. PMID 27261791.
- ^ Jump up to: a b Klaile Y, Schlack K, Boegemann M, Steinestel J, Schrader AJ, Krabbe LM (October 2016). "Variant histology in bladder cancer: how it should change the management in non-muscle invasive and muscle invasive disease?". Translational Andrology and Urology. 5 (5): 692–701. doi:10.21037/tau.2016.06.13. PMC 5071184. PMID 27785426.
- ^ Abeloff's clinical oncology. Niederhuber, John E.,, Armitage, James O., 1946-, Doroshow, James H.,, Kastan, M. B. (Michael B.),, Tepper, Joel E.,, Preceded by: Abeloff, Martin D. (6th ed.). Philadelphia, PA. 8 January 2019. p. 1388. ISBN 978-0-323-56815-9. OCLC 1089396489.CS1 maint: others (link)
- ^ Hodges, Stephanie C.; Holt, Harry R.; Degeorge, Katharine C. (15 October 2017). "Bladder Cancer: Diagnosis and Treatment". American Family Physician. 96 (8): 507–514. PMID 29094888.
- ^ Zeegers MP, Tan FE, Dorant E, van Den Brandt PA (August 2000). "The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies". Cancer. 89 (3): 630–9. doi:10.1002/1097-0142(20000801)89:3<630::AID-CNCR19>3.0.CO;2-Q. PMID 10931463.
- ^ Jump up to: a b van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP (June 2016). "Quantified relations between exposure to tobacco smoking and bladder cancer risk: a meta-analysis of 89 observational studies". International Journal of Epidemiology. 45 (3): 857–70. doi:10.1093/ije/dyw044. PMID 27097748.
- ^ Letašiová S, Medve'ová A, Šovčíková A, Dušinská M, Volkovová K, Mosoiu C, Bartonová A (June 2012). "Bladder cancer, a review of the environmental risk factors". Environmental Health. 11 Suppl 1: S11. doi:10.1186/1476-069X-11-S1-S11. PMC 3388449. PMID 22759493.
- ^ Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, et al. (April 2000). "Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies". International Journal of Cancer. 86 (2): 289–94. doi:10.1002/(sici)1097-0215(20000415)86:2<289::aid-ijc21>3.0.co;2-m. PMID 10738259.
- ^ Yan H, Ying Y, Xie H, Li J, Wang X, He L, et al. (2018). "Secondhand smoking increases bladder cancer risk in nonsmoking population: a meta-analysis". Cancer Management and Research. 10: 3781–3791. doi:10.2147/CMAR.S175062. PMC 6159806. PMID 30288109.
- ^ "Health Risks of Secondhand Smoke". www.cancer.org. Retrieved 21 November 2019.
- ^ Afshari M, Janbabaei G, Bahrami MA, Moosazadeh M (2017). "Opium and bladder cancer: A systematic review and meta-analysis of the odds ratios for opium use and the risk of bladder cancer". PLOS ONE. 12 (6): e0178527. Bibcode:2017PLoSO..1278527A. doi:10.1371/journal.pone.0178527. PMC 5460843. PMID 28586371.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risk to Humans (2012). 4-AMINOBIPHENYL. International Agency for Research on Cancer.
- ^ Saint-Jacques N, Parker L, Brown P, Dummer TJ (June 2014). "Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence". Environmental Health. 13: 44. doi:10.1186/1476-069X-13-44. PMC 4088919. PMID 24889821.
- ^ Clin B, Pairon JC (November 2014). "Medical follow-up for workers exposed to bladder carcinogens: the French evidence-based and pragmatic statement". BMC Public Health. 14: 1155. doi:10.1186/1471-2458-14-1155. PMC 4230399. PMID 25377503.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risk to Humans (2012). CHLORNAPHAZINE. International Agency for Research on Cancer.
- ^ Humans, IARC Working Group on the Evaluation of Carcinogenic Risk to (2012). 2-NAPHTHYLAMINE. International Agency for Research on Cancer.
- ^ Reulen RC, Kellen E, Buntinx F, Brinkman M, Zeegers MP (September 2008). "A meta-analysis on the association between bladder cancer and occupation". Scandinavian Journal of Urology and Nephrology. Supplementum. 42 (218): 64–78. doi:10.1080/03008880802325192. PMID 18815919. S2CID 30510231.
- ^ Guha N, Steenland NK, Merletti F, Altieri A, Cogliano V, Straif K (August 2010). "Bladder cancer risk in painters: a meta-analysis". Occupational and Environmental Medicine. 67 (8): 568–73. doi:10.1136/oem.2009.051565. PMID 20647380.
- ^ Harling M, Schablon A, Schedlbauer G, Dulon M, Nienhaus A (May 2010). "Bladder cancer among hairdressers: a meta-analysis". Occupational and Environmental Medicine. 67 (5): 351–8. doi:10.1136/oem.2009.050195. PMC 2981018. PMID 20447989.
- ^ Mostafa MH, Sheweita SA, O'Connor PJ (January 1999). "Relationship between schistosomiasis and bladder cancer". Clinical Microbiology Reviews. 12 (1): 97–111. doi:10.1128/CMR.12.1.97. PMC 88908. PMID 9880476.
- ^ Zaghloul MS (December 2012). "Bladder cancer and schistosomiasis". Journal of the Egyptian National Cancer Institute. 24 (4): 151–9. doi:10.1016/j.jnci.2012.08.002. PMID 23159285.
- ^ Mostafa MH, Helmi S, Badawi AF, Tricker AR, Spiegelhalder B, Preussmann R (April 1994). "Nitrate, nitrite and volatile N-nitroso compounds in the urine of Schistosoma haematobium and Schistosoma mansoni infected patients". Carcinogenesis. 15 (4): 619–25. doi:10.1093/carcin/15.4.619. PMID 8149471.
- ^ Badawi AF (August 1996). "Molecular and genetic events in schistosomiasis-associated human bladder cancer: role of oncogenes and tumor suppressor genes". Cancer Letters. 105 (2): 123–38. doi:10.1016/0304-3835(96)04284-x. PMID 8697435.
- ^ Chaudhary KS, Lu QL, Abel PD, Khandan-Nia N, Shoma AM, el Baz M, et al. (January 1997). "Expression of bcl-2 and p53 oncoproteins in schistosomiasis-associated transitional and squamous cell carcinoma of urinary bladder". British Journal of Urology. 79 (1): 78–84. doi:10.1046/j.1464-410x.1997.30717.x. PMID 9043502.
- ^ Shokeir AA (January 2004). "Squamous cell carcinoma of the bladder: pathology, diagnosis and treatment". BJU International. 93 (2): 216–20. doi:10.1111/j.1464-410x.2004.04588.x. PMID 14690486. S2CID 10487371.
- ^ Monach PA, Arnold LM, Merkel PA (January 2010). "Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: a data-driven review". Arthritis and Rheumatism. 62 (1): 9–21. doi:10.1002/art.25061. PMID 20039416.
- ^ Yang HY, Chen PC, Wang JD (2014). "Chinese herbs containing aristolochic acid associated with renal failure and urothelial carcinoma: a review from epidemiologic observations to causal inference". BioMed Research International. 2014: 569325. doi:10.1155/2014/569325. PMC 4241283. PMID 25431765.
- ^ Suriano F, Altobelli E, Sergi F, Buscarini M (2013). "Bladder cancer after radiotherapy for prostate cancer". Reviews in Urology. 15 (3): 108–12. PMC 3821989. PMID 24223022.
- ^ Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB (24 March 2015). "Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies". PLOS ONE. 10 (3): e0119313. Bibcode:2015PLoSO..1019313S. doi:10.1371/journal.pone.0119313. PMC 4372289. PMID 25803438.
- ^ Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP (September 2016). "Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses". European Journal of Epidemiology. 31 (9): 811–51. doi:10.1007/s10654-016-0138-6. PMC 5010611. PMID 27000312.
- ^ "Cancer Genetics Browser". cancer.sanger.ac.uk. Retrieved 21 November 2019.
- ^ Online Mendelian Inheritance in Man (OMIM): 109800
- ^ Zhang X, Zhang Y (September 2015). "Bladder Cancer and Genetic Mutations". Cell Biochemistry and Biophysics. 73 (1): 65–9. doi:10.1007/s12013-015-0574-z. PMID 27352265. S2CID 14316154.
- ^ "Bladder cancer". Genetics Home Reference.
- ^ Ahmad I, Sansom OJ, Leung HY (May 2012). "Exploring molecular genetics of bladder cancer: lessons learned from mouse models". Disease Models & Mechanisms. 5 (3): 323–32. doi:10.1242/dmm.008888. PMC 3339826. PMID 22422829.
- ^ Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE (July 2016). "The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours" (PDF). European Urology. 70 (1): 106–119. doi:10.1016/j.eururo.2016.02.028. PMID 26996659.
- ^ Engel LS, Taioli E, Pfeiffer R, Garcia-Closas M, Marcus PM, Lan Q, et al. (July 2002). "Pooled analysis and meta-analysis of glutathione S-transferase M1 and bladder cancer: a HuGE review". American Journal of Epidemiology. 156 (2): 95–109. doi:10.1093/aje/kwf018. PMID 12117698.
- ^ Jump up to: a b c "Bladder Cancer Report" (PDF). World Cancer Research Fund : International. Retrieved 9 November 2019.
- ^ Jump up to: a b c Marks P, Soave A, Shariat SF, Fajkovic H, Fisch M, Rink M (October 2016). "Female with bladder cancer: what and why is there a difference?". Translational Andrology and Urology. 5 (5): 668–682. doi:10.21037/tau.2016.03.22. PMC 5071204. PMID 27785424.
- ^ Choi W, Ochoa A, McConkey DJ, Aine M, Höglund M, Kim WY, et al. (September 2017). "Genetic Alterations in the Molecular Subtypes of Bladder Cancer: Illustration in the Cancer Genome Atlas Dataset". European Urology. 72 (3): 354–365. doi:10.1016/j.eururo.2017.03.010. PMC 5764190. PMID 28365159.
- ^ Choi W, Czerniak B, Ochoa A, Su X, Siefker-Radtke A, Dinney C, McConkey DJ (July 2014). "Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer". Nature Reviews. Urology. 11 (7): 400–10. doi:10.1038/nrurol.2014.129. PMID 24960601. S2CID 24723395.
- ^ "EAU Guidelines: Non-muscle-invasive Bladder Cancer Diagnosis". Uroweb. Retrieved 12 November 2019.
- ^ Jump up to: a b "EAU Guidelines: Muscle-invasive and Metastatic Bladder Cancer diagnosis". Uroweb. Retrieved 12 November 2019.
- ^ Jump up to: a b c "Uroweb - European Association of Urology (EAU)". Uroweb. Retrieved 7 November 2019.
- ^ "Bladder Cancer Treatment". National Cancer Institute. 8 May 2020. Retrieved 4 June 2020.
- ^ Lotan Y, Roehrborn CG (January 2003). "Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses". Urology. 61 (1): 109–18, discussion 118. doi:10.1016/S0090-4295(02)02136-2. PMID 12559279.
- ^ Shariat SF, Karam JA, Lotan Y, Karakiewizc PI (2008). "Critical evaluation of urinary markers for bladder cancer detection and monitoring". Reviews in Urology. 10 (2): 120–35. PMC 2483317. PMID 18660854.
- ^ Jump up to: a b Chou R, Gore JL, Buckley D, Fu R, Gustafson K, Griffin JC, et al. (December 2015). "Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis". Annals of Internal Medicine. 163 (12): 922–31. doi:10.7326/M15-0997. PMID 26501851.
- ^ Santoni G, Morelli MB, Amantini C, Battelli N (2018). "Urinary Markers in Bladder Cancer: An Update". Frontiers in Oncology. 8: 362. doi:10.3389/fonc.2018.00362. PMC 6137202. PMID 30245975.
- ^ Jump up to: a b c Miyake M, Owari T, Hori S, Nakai Y, Fujimoto K (2018). "Emerging biomarkers for the diagnosis and monitoring of urothelial carcinoma". Research and Reports in Urology. 10: 251–261. doi:10.2147/RRU.S173027. PMC 6299471. PMID 30588457.
- ^ Jump up to: a b Goodison S, Rosser CJ, Urquidi V (April 2013). "Bladder cancer detection and monitoring: assessment of urine- and blood-based marker tests". Molecular Diagnosis & Therapy. 17 (2): 71–84. doi:10.1007/s40291-013-0023-x. PMC 3627848. PMID 23479428.
- ^ Health, Center for Devices and Radiological (7 October 2019). "Nucleic Acid Based Tests". FDA.
- ^ Soria F, Droller MJ, Lotan Y, Gontero P, D'Andrea D, Gust KM, et al. (December 2018). "An up-to-date catalog of available urinary biomarkers for the surveillance of non-muscle invasive bladder cancer". World Journal of Urology. 36 (12): 1981–1995. doi:10.1007/s00345-018-2380-x. PMC 6280823. PMID 29931526.
- ^ Jump up to: a b c d Andreassen, B. K.; Aagnes, B.; Gislefoss, R.; Andreassen, M.; Wahlqvist, R. (2016). "Incidence and Survival of urothelial carcinoma of the urinary bladder in Norway 1981-2014". BMC Cancer. 16 (1): 799. doi:10.1186/s12885-016-2832-x. ISSN 1471-2407. PMC 5064906. PMID 27737647.
- ^ Jump up to: a b c "Types of Bladder Cancer: TCC & Other Variants". CancerCenter.com. Retrieved 10 August 2018.
- ^ Jump up to: a b Amin MB (June 2009). "Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications". Modern Pathology. 22 Suppl 2 (S2): S96–S118. doi:10.1038/modpathol.2009.26. PMID 19494856.
- ^ Tang DH, Chang SS (December 2015). "Management of carcinoma in situ of the bladder: best practice and recent developments". Therapeutic Advances in Urology. 7 (6): 351–64. doi:10.1177/1756287215599694. PMC 4647140. PMID 26622320.
- ^ Chalasani V, Chin JL, Izawa JI (December 2009). "Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer". Canadian Urological Association Journal. 3 (6 Suppl 4): S193-8. doi:10.5489/cuaj.1195. PMC 2792446. PMID 20019984.
- ^ Moschini M, D'Andrea D, Korn S, Irmak Y, Soria F, Compérat E, Shariat SF (November 2017). "Characteristics and clinical significance of histological variants of bladder cancer". Nature Reviews. Urology. 14 (11): 651–668. doi:10.1038/nrurol.2017.125. PMID 28895563. S2CID 6351401.
- ^ Warrick JI (October 2017). "Clinical Significance of Histologic Variants of Bladder Cancer". Journal of the National Comprehensive Cancer Network. 15 (10): 1268–1274. doi:10.6004/jnccn.2017.7027. PMID 28982751.
- ^ Venyo AK, Titi S (2014). "Sarcomatoid variant of urothelial carcinoma (carcinosarcoma, spindle cell carcinoma): a review of the literature". ISRN Urology. 2014: 794563. doi:10.1155/2014/794563. PMC 3920806. PMID 24587922.
- ^ "Urothelial Carcinoma Variants - American Urological Association". www.auanet.org.
- ^ "EAU Guidelines - STAGING AND CLASSIFICATION SYSTEMS". Uroweb.
- ^ Magers MJ, Lopez-Beltran A, Montironi R, Williamson SR, Kaimakliotis HZ, Cheng L (January 2019). "Staging of bladder cancer". Histopathology. 74 (1): 112–134. doi:10.1111/his.13734. PMID 30565300.
- ^ Jump up to: a b "Bladder Cancer: Stages and Grades". Cancer.net. Approved by the Cancer.Net Editorial Board 05/2019
- ^ Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD (January 2011). "Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor". AJR. American Journal of Roentgenology. 196 (1): 117–22. doi:10.2214/AJR.10.5036. PMID 21178055.
- ^ Jump up to: a b Mao Y, Hedgire S, Prapruttam D, Harisinghani M (16 September 2014). "Imaging of Pelvic Lymph Nodes". Current Radiology Reports. 2 (11). doi:10.1007/s40134-014-0070-z.
- ^ Shankar PR, Barkmeier D, Hadjiiski L, Cohan RH (October 2018). "A pictorial review of bladder cancer nodal metastases". Translational Andrology and Urology. 7 (5): 804–813. doi:10.21037/tau.2018.08.25. PMC 6212631. PMID 30456183.
- ^ "How is bladder cancer staged?". American Cancer Society. Archived from the original on 4 October 2015. Last Medical Review: 11/02/2019
- ^ "Survival rates for bladder cancer by stage". American Cancer Society. Archived from the original on 13 October 2015. Last Medical Review: 02/26/2014
- ^ Seth P. Lerner. "Overview of Diagnosis and Management of Non-Muscle Invasive Bladder Cancer" (PDF). Food and Drug Administration. ODAC 14 September 2016
- ^ Epstein JI, Amin MB, Reuter VR, Mostofi FK (December 1998). "The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee". The American Journal of Surgical Pathology. 22 (12): 1435–48. doi:10.1097/00000478-199812000-00001. PMID 9850170.
- ^ Compérat EM, Burger M, Gontero P, Mostafid AH, Palou J, Rouprêt M, et al. (May 2019). "Grading of Urothelial Carcinoma and The New "World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016"". European Urology Focus. 5 (3): 457–466. doi:10.1016/j.euf.2018.01.003. PMID 29366854.
- ^ Jump up to: a b Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. (October 2016). "Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline". The Journal of Urology. 196 (4): 1021–9. doi:10.1016/j.juro.2016.06.049. PMID 27317986.
- ^ Jump up to: a b "Bladder Cancer: Non-Muscle Invasive Guideline - American Urological Association". www.auanet.org.
- ^ Soukup V, Čapoun O, Cohen D, Hernández V, Burger M, Compérat E, et al. (November 2018). "Risk Stratification Tools and Prognostic Models in Non-muscle-invasive Bladder Cancer: A Critical Assessment from the European Association of Urology Non-muscle-invasive Bladder Cancer Guidelines Panel". European Urology Focus. 6 (3): 479–489. doi:10.1016/j.euf.2018.11.005. PMID 30470647.
- ^ Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. (March 2006). "Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials". European Urology. 49 (3): 466–5, discussion 475–7. doi:10.1016/j.eururo.2005.12.031. PMID 16442208.
- ^ Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. (March 2006). "Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials". European Urology. 49 (3): 466–5, discussion 475–7. doi:10.1016/j.eururo.2005.12.031. PMID 16442208.
- ^ Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, et al. (November 2009). "Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model". The Journal of Urology. 182 (5): 2195–203. doi:10.1016/j.juro.2009.07.016. PMID 19758621.
- ^ "EAU Guidelines: Non-muscle-invasive Bladder Cancer". Uroweb.
- ^ Choi SY, Ryu JH, Chang IH, Kim TH, Myung SC, Moon YT, et al. (October 2014). "Predicting recurrence and progression of non-muscle-invasive bladder cancer in Korean patients: a comparison of the EORTC and CUETO models". Korean Journal of Urology. 55 (10): 643–9. doi:10.4111/kju.2014.55.10.643. PMC 4198762. PMID 25324946.
- ^ Jump up to: a b Brinkman M, Zeegers MP (September 2008). "Nutrition, total fluid and bladder cancer". Scandinavian Journal of Urology and Nephrology. Supplementum. 42 (218): 25–36. doi:10.1080/03008880802285073. PMID 18815914. S2CID 21780999.
- ^ Brinkman M, Buntinx F, Muls E, Zeegers MP (September 2006). "Use of selenium in chemoprevention of bladder cancer". The Lancet. Oncology. 7 (9): 766–74. doi:10.1016/S1470-2045(06)70862-2. PMID 16945772.
- ^ Zamora-Ros R, Sacerdote C, Ricceri F, Weiderpass E, Roswall N, Buckland G, et al. (October 2014). "Flavonoid and lignan intake in relation to bladder cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study". British Journal of Cancer. 111 (9): 1870–80. doi:10.1038/bjc.2014.459. PMC 4453722. PMID 25121955.
- ^ Valtin H (November 2002). ""Drink at least eight glasses of water a day." Really? Is there scientific evidence for "8 x 8"?". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 283 (5): R993-1004. doi:10.1152/ajpregu.00365.2002. PMID 12376390. S2CID 2256436.
- ^ "Final Update Summary: Bladder Cancer in Adults: Screening - US Preventive Services Task Force". www.uspreventiveservicestaskforce.org. Retrieved 13 November 2019.
- ^ Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, et al. (December 2016). "Bladder cancer". Lancet. 388 (10061): 2796–2810. doi:10.1016/S0140-6736(16)30512-8. PMID 27345655. S2CID 29104789.
- ^ Jump up to: a b c "NCCN Bladder cancer guidelines 2018" (PDF). Retrieved 25 November 2019.
- ^ Witjes JA, Babjuk M, Gontero P, Jacqmin D, Karl A, Kruck S, et al. (November 2014). "Clinical and cost effectiveness of hexaminolevulinate-guided blue-light cystoscopy: evidence review and updated expert recommendations" (PDF). European Urology. 66 (5): 863–71. doi:10.1016/j.eururo.2014.06.037. PMID 25001887.
- ^ Daneshmand S, Schuckman AK, Bochner BH, Cookson MS, Downs TM, Gomella LG, et al. (October 2014). "Hexaminolevulinate blue-light cystoscopy in non-muscle-invasive bladder cancer: review of the clinical evidence and consensus statement on appropriate use in the USA". Nature Reviews. Urology. 11 (10): 589–96. doi:10.1038/nrurol.2014.245. PMID 25245244.
- ^ Miladi M, Peyromaure M, Zerbib M, Saïghi D, Debré B (March 2003). "The value of a second transurethral resection in evaluating patients with bladder tumours". European Urology. 43 (3): 241–5. doi:10.1016/s0302-2838(03)00040-x. PMID 12600426.
- ^ Jump up to: a b "EAU Guidelines: Non-muscle-invasive Bladder Cancer". Uroweb.
- ^ Jump up to: a b c Schmidt, Stefanie; Kunath, Frank; Coles, Bernadette; Draeger, Desiree Louise; Krabbe, Laura-Maria; Dersch, Rick; Kilian, Samuel; Jensen, Katrin; Dahm, Philipp; Meerpohl, Joerg J (8 January 2020). Cochrane Urology Group (ed.). "Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer". Cochrane Database of Systematic Reviews. 1: CD011935. doi:10.1002/14651858.CD011935.pub2. PMC 6956215. PMID 31912907.
- ^ Jump up to: a b Zamboni S, Baumeister P, Mattei A, Mordasini L, Antonelli A, Simeone C, Moschini M (February 2019). "Single postoperative instillation for non-muscle invasive bladder cancer: are there still any indication?". Translational Andrology and Urology. 8 (1): 76–84. doi:10.21037/tau.2018.08.20. PMC 6414349. PMID 30976571.
- ^ Witjes JA, Hendricksen K (January 2008). "Intravesical pharmacotherapy for non-muscle-invasive bladder cancer: a critical analysis of currently available drugs, treatment schedules, and long-term results". European Urology. 53 (1): 45–52. doi:10.1016/j.eururo.2007.08.015. PMID 17719169.
- ^ Di Stasi SM, Riedl C (June 2009). "Updates in intravesical electromotive drug administration of mitomycin-C for non-muscle invasive bladder cancer" (PDF). World Journal of Urology. 27 (3): 325–30. doi:10.1007/s00345-009-0389-x. hdl:2108/6440. PMID 19234707. S2CID 24496739.
- ^ Kos B, Vásquez JL, Miklavčič D, Hermann GG, Gehl J (2016). "Investigation of the mechanisms of action behind Electromotive Drug Administration (EMDA)". PeerJ. 4 (e2309): e2309. doi:10.7717/peerj.2309. PMC 5012313. PMID 27635313.
- ^ Bahouth Z, Halachmi S, Moskovitz B, Nativ O (2016). "The role of hyperthermia as a treatment for non-muscle invasive bladder cancer". Expert Review of Anticancer Therapy. 16 (2): 189–98. doi:10.1586/14737140.2016.1126515. PMID 26618756. S2CID 681090.
- ^ Alexandroff AB, Jackson AM, O'Donnell MA, James K (May 1999). "BCG immunotherapy of bladder cancer: 20 years on". Lancet. 353 (9165): 1689–94. doi:10.1016/S0140-6736(98)07422-4. PMID 10335805. S2CID 19355109.
- ^ Lamm DL, Blumenstein BA, Crawford ED, Montie JE, Scardino P, Grossman HB, et al. (October 1991). "A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder". The New England Journal of Medicine. 325 (17): 1205–9. doi:10.1056/NEJM199110243251703. PMID 1922207.
- ^ Kuroda K, Brown EJ, Telle WB, Russell DG, Ratliff TL (January 1993). "Characterization of the internalization of bacillus Calmette-Guerin by human bladder tumor cells". The Journal of Clinical Investigation. 91 (1): 69–76. doi:10.1172/JCI116202. PMC 329996. PMID 8423234.
- ^ Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ (September 1993). "T-cell subsets required for intravesical BCG immunotherapy for bladder cancer". The Journal of Urology. 150 (3): 1018–23. doi:10.1016/s0022-5347(17)35678-1. PMID 8102183.
- ^ Fuge O, Vasdev N, Allchorne P, Green JS (2015). "Immunotherapy for bladder cancer". Research and Reports in Urology. 7: 65–79. doi:10.2147/RRU.S63447. PMC 4427258. PMID 26000263.
- ^ Jump up to: a b c d Kamat AM, Flaig TW, Grossman HB, Konety B, Lamm D, O'Donnell MA, et al. (April 2015). "Expert consensus document: Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer". Nature Reviews. Urology. 12 (4): 225–35. doi:10.1038/nrurol.2015.58. PMID 25800393.
- ^ Alhunaidi O, Zlotta AR (2019). "The use of intravesical BCG in urothelial carcinoma of the bladder". ecancermedicalscience. 13: 905. doi:10.3332/ecancer.2019.905. PMC 6411413. PMID 30915163.
- ^ D'Andrea D, Gontero P, Shariat SF, Soria F (February 2019). "Intravesical bacillus Calmette-Guérin for bladder cancer: are all the strains equal?". Translational Andrology and Urology. 8 (1): 85–93. doi:10.21037/tau.2018.08.19. PMC 6414340. PMID 30976572.
- ^ Macleod LC, Ngo TC, Gonzalgo ML (July 2014). "Complications of intravesical bacillus calmette-guérin". Canadian Urological Association Journal. 8 (7–8): E540-4. doi:10.5489/cuaj.1411. PMC 4137021. PMID 25210559.
- ^ Shah S, Carter-Monroe N, Atta MG (October 2015). "Granulomatous interstitial nephritis". Clinical Kidney Journal. 8 (5): 516–23. doi:10.1093/ckj/sfv053. PMC 4581373. PMID 26413275.
- ^ Decaestecker K, Oosterlinck W (2015). "Managing the adverse events of intravesical bacillus Calmette-Guérin therapy". Research and Reports in Urology. 7: 157–63. doi:10.2147/RRU.S63448. PMC 4630183. PMID 26605208.
- ^ Witjes JA (May 2006). "Management of BCG failures in superficial bladder cancer: a review". European Urology. 49 (5): 790–7. doi:10.1016/j.eururo.2006.01.017. PMID 16464532.
- ^ Babjuk W, Oosterlinck W, Sylvester R, et al. (2010). "Guidelines on TaT1 (Non-muscle invasive) Bladder Cancer". European Association of Urology. Archived from the original on 24 April 2010.
- ^ Bladder Cancer Clinical Guideline Update Panel (2007). Bladder Cancer: Guideline for the Management of Nonmuscle Invasive Bladder Cancer: (Stages Ta, T1, and Tis): 2007 Update. American Urological Association.[page needed]
- ^ Hassler MR, Shariat SF, Soria F (May 2019). "Salvage therapeutic strategies for bacillus Calmette-Guerin failure". Current Opinion in Urology. 29 (3): 239–246. doi:10.1097/MOU.0000000000000593. PMID 30762670. S2CID 73439134.
- ^ Jump up to: a b c d "Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline (2017) - American Urological Association". www.auanet.org. Retrieved 20 November 2019.
- ^ Jump up to: a b c Hwang, Eu Chang; Sathianathen, Niranjan J; Imamura, Mari; Kuntz, Gretchen M; Risk, Michael C; Dahm, Philipp (14 May 2019). Cochrane Urology Group (ed.). "Extended versus standard lymph node dissection for urothelial carcinoma of the bladder in patients undergoing radical cystectomy". Cochrane Database of Systematic Reviews. 5: CD013336. doi:10.1002/14651858.CD013336. PMC 6528183. PMID 31111956.
- ^ Johar RS, Hayn MH, Stegemann AP, Ahmed K, Agarwal P, Balbay MD, et al. (July 2013). "Complications after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium". European Urology. 64 (1): 52–7. doi:10.1016/j.eururo.2013.01.010. PMID 23380164.
- ^ Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. (January 2009). "Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology". European Urology. 55 (1): 164–74. doi:10.1016/j.eururo.2008.07.031. PMID 18675501.
- ^ Moschini M, Simone G, Stenzl A, Gill IS, Catto J (April 2016). "Critical Review of Outcomes from Radical Cystectomy: Can Complications from Radical Cystectomy Be Reduced by Surgical Volume and Robotic Surgery?". European Urology Focus. 2 (1): 19–29. doi:10.1016/j.euf.2016.03.001. PMID 28723446.
- ^ Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, et al. (December 2013). "Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(®)) society recommendations". Clinical Nutrition. 32 (6): 879–87. doi:10.1016/j.clnu.2013.09.014. PMID 24189391.
- ^ "UpToDate: Neoadjuvant chemotherapy". www.uptodate.com.
- ^ Vale, C. (June 2003). "Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis". Lancet. 361 (9373): 1927–34. doi:10.1016/s0140-6736(03)13580-5. PMID 12801735. S2CID 29233494.
- ^ Advanced Bladder Cancer (ABC) Meta-analysis Collaboration (August 2005). "Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration". European Urology. 48 (2): 202–5, discussion 205–6. doi:10.1016/j.eururo.2005.04.006. PMID 15939524.
- ^ Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. (August 2003). "Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer". The New England Journal of Medicine. 349 (9): 859–66. doi:10.1056/NEJMoa022148. PMID 12944571.
- ^ Jump up to: a b Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. (June 2016). "Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis". The Oncologist. 21 (6): 708–15. doi:10.1634/theoncologist.2015-0440. PMC 4912364. PMID 27053504.
- ^ Iwata T, Kimura S, Abufaraj M, Janisch F, Karakiewicz PI, Seebacher V, et al. (October 2019). "The role of adjuvant radiotherapy after surgery for upper and lower urinary tract urothelial carcinoma: A systematic review". Urologic Oncology. 37 (10): 659–671. doi:10.1016/j.urolonc.2019.05.021. PMID 31255542.
- ^ Jump up to: a b c d "Bladder Cancer Treatment Regimens". Cancer Therapy Advisor. 1 January 2019. Retrieved 26 November 2019.
- ^ "UpToDate". www.uptodate.com. Retrieved 26 November 2019.
- ^ Jump up to: a b c Ploussard G, Daneshmand S, Efstathiou JA, Herr HW, James ND, Rödel CM, et al. (July 2014). "Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review". European Urology. 66 (1): 120–37. doi:10.1016/j.eururo.2014.02.038. PMID 24613684.
- ^ Smelser WW, Austenfeld MA, Holzbeierlein JM, Lee EK. Where are we with bladder preservation for muscle-invasive bladder cancer in 2017?. Indian J Urol 2017;33:111–7 http://www.indianjurol.com/text.asp?2017/33/2/111/203415 Archived 10 September 2017 at the Wayback Machine
- ^ Mirza A, Choudhury A (April 2016). "Bladder Preservation for Muscle Invasive Bladder Cancer". Bladder Cancer. 2 (2): 151–163. doi:10.3233/BLC-150025. PMC 4927909. PMID 27376137.
- ^ "Chemotherapy for Bladder Cancer". www.cancer.org. Retrieved 27 November 2019.
- ^ "EAU Guidelines: Muscle-invasive and Metastatic Bladder Cancer". Uroweb.
- ^ Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. (June 2011). "Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy". Journal of Clinical Oncology. 29 (17): 2432–8. doi:10.1200/JCO.2011.34.8433. PMID 21555688. S2CID 33263969.
- ^ Jump up to: a b Keam B (2018). "Section VI. Chemotherapy for Metastatic Bladder Cancer". Bladder Cancer. Academic Press. p. 527. ISBN 978-0-12-809939-1. Retrieved 27 November 2019.
- ^ Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM, et al. (October 2013). "The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials". Annals of Oncology. 24 (10): 2475–84. doi:10.1093/annonc/mdt226. PMC 3841419. PMID 23788754.
- ^ Gilligan TD, Steele GS, Zietman AL, Kantoff PW (2003). Chemotherapy for Metastatic Disease.
- ^ Galsky MD, Chen GJ, Oh WK, Bellmunt J, Roth BJ, Petrioli R, et al. (February 2012). "Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma". Annals of Oncology. 23 (2): 406–10. doi:10.1093/annonc/mdr156. PMID 21543626.
- ^ Shelley MD, Cleves A, Wilt TJ, Mason MD (July 2011). "Gemcitabine chemotherapy for the treatment of metastatic bladder carcinoma". BJU International. 108 (2): 168–79. doi:10.1111/j.1464-410X.2011.10341.x. PMID 21718430. S2CID 26003400.
- ^ Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, et al. (March 2008). "Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel". Annals of Oncology. 19 (3): 420–32. doi:10.1093/annonc/mdm442. PMID 17906299.
- ^ Sridhar SS (May 2017). "Evolving Treatment of Advanced Urothelial Cancer". Journal of Oncology Practice. 13 (5): 309–315. doi:10.1200/JOP.2017.022137. PMID 28489981.
- ^ Gerullis H, Wawroschek F, Köhne CH, Ecke TH (January 2017). "Vinflunine in the treatment of advanced urothelial cancer: clinical evidence and experience". Therapeutic Advances in Urology. 9 (1): 28–35. doi:10.1177/1756287216677903. PMC 5167074. PMID 28042310.
- ^ Oing C, Rink M, Oechsle K, Seidel C, von Amsberg G, Bokemeyer C (February 2016). "Second Line Chemotherapy for Advanced and Metastatic Urothelial Carcinoma: Vinflunine and Beyond-A Comprehensive Review of the Current Literature". The Journal of Urology. 195 (2): 254–63. doi:10.1016/j.juro.2015.06.115. PMID 26410730.
- ^ Keam B (2018). "Section VI. Chemotherapy for Metastatic Bladder Cancer". Bladder Cancer. Academic Press. p. 530. ISBN 978-0-12-809939-1. Retrieved 27 November 2019.
- ^ "Erdafitinib Effective Against Advanced Bladder Cancer". National Cancer Institute. 9 August 2019.
- ^ Konala VM, Adapa S, Aronow WS (February 2019). "Immunotherapy in Bladder Cancer". American Journal of Therapeutics: 1. doi:10.1097/MJT.0000000000000934. PMID 30839322. S2CID 73472390.
- ^ Katz H, Wassie E, Alsharedi M (September 2017). "Checkpoint inhibitors: the new treatment paradigm for urothelial bladder cancer". Medical Oncology. 34 (10): 170. doi:10.1007/s12032-017-1029-8. PMID 28864844. S2CID 22280860.
- ^ Jump up to: a b c Narayan, Vikram; Kahlmeyer, Andreas; Dahm, Philipp; Skoetz, Nicole; Risk, Michael C; Bongiorno, Connie; Patel, Neil; Hwang, Eu Chang; Jung, Jae Hung; Gartlehner, Gerald; Kunath, Frank (23 July 2018). Cochrane Urology Group (ed.). "Pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy. A Cochrane Rapid Review". Cochrane Database of Systematic Reviews. 7: CD012838. doi:10.1002/14651858.CD012838.pub2. PMC 6513246. PMID 30036453.
- ^ Heidenreich A, Albers P, Classen J, Graefen M, Gschwend J, Kotzerke J, et al. (2010). "Imaging studies in metastatic urogenital cancer patients undergoing systemic therapy: recommendations of a multidisciplinary consensus meeting of the Association of Urological Oncology of the German Cancer Society". Urologia Internationalis. 85 (1): 1–10. doi:10.1159/000318985. PMID 20693823.
- ^ Llewelyn R. "Response evaluation criteria in solid tumors | Radiology Reference Article | Radiopaedia.org". Radiopaedia.
- ^ Siddiqui MR, Grant C, Sanford T, Agarwal PK (August 2017). "Current clinical trials in non-muscle invasive bladder cancer". Urologic Oncology. 35 (8): 516–527. doi:10.1016/j.urolonc.2017.06.043. PMC 5556973. PMID 28778250.
- ^ "Bladder Cancer - Statistics". Cancer.Net. 25 June 2012.
- ^ Kaufman DS, Shipley WU, Feldman AS (July 2009). "Bladder cancer". Lancet. 374 (9685): 239–49. doi:10.1016/S0140-6736(09)60491-8. PMID 19520422. S2CID 40417997.
- ^ van der Heijden AG, Witjes JA (September 2009). "Recurrence, Progression, and Follow-Up in Non–Muscle-Invasive Bladder Cancer". European Urology Supplements. 8 (7): 556–562. doi:10.1016/j.eursup.2009.06.010.
- ^ Tyson MD, Chang SS, Keegan KA (2016). "Role of consolidative surgical therapy in patients with locally advanced or regionally metastatic bladder cancer". Bladder. 3 (2). doi:10.14440/bladder.2016.89 (inactive 31 May 2021). PMC 5336315. PMID 28261632.CS1 maint: DOI inactive as of May 2021 (link)
- ^ Zhang L, Wu B, Zha Z, Qu W, Zhao H, Yuan J (July 2019). "Clinicopathological factors in bladder cancer for cancer-specific survival outcomes following radical cystectomy: a systematic review and meta-analysis". BMC Cancer. 19 (1): 716. doi:10.1186/s12885-019-5924-6. PMC 6642549. PMID 31324162.
- ^ Ku JH, Kang M, Kim HS, Jeong CW, Kwak C, Kim HH (June 2015). "Lymph node density as a prognostic variable in node-positive bladder cancer: a meta-analysis". BMC Cancer. 15: 447. doi:10.1186/s12885-015-1448-x. PMC 4450458. PMID 26027955.
- ^ Yang LS, Shan BL, Shan LL, Chin P, Murray S, Ahmadi N, Saxena A (September 2016). "A systematic review and meta-analysis of quality of life outcomes after radical cystectomy for bladder cancer". Surgical Oncology. 25 (3): 281–97. doi:10.1016/j.suronc.2016.05.027. PMID 27566035.
- ^ Somani BK, Gimlin D, Fayers P, N'dow J (November 2009). "Quality of life and body image for bladder cancer patients undergoing radical cystectomy and urinary diversion--a prospective cohort study with a systematic review of literature". Urology. 74 (5): 1138–43. doi:10.1016/j.urology.2009.05.087. PMID 19773042.
- ^ Vartolomei L, Ferro M, Mirone V, Shariat SF, Vartolomei MD (July 2018). "Systematic Review: Depression and Anxiety Prevalence in Bladder Cancer Patients". Bladder Cancer. 4 (3): 319–326. doi:10.3233/BLC-180181. PMC 6087432. PMID 30112443.
- ^ David E (February 2017). "Psychiatric disorders worsen bladder cancer survival". www.healio.com. Retrieved 27 November 2019.
- ^ Correa AF, Smaldone MC (August 2018). "Melancholia and cancer: The bladder cancer narrative". Cancer. 124 (15): 3080–3083. doi:10.1002/cncr.31402. PMID 29660788.
- ^ "Bladder cancer statistics". World Cancer Research Fund. 22 August 2018. Retrieved 9 November 2019.
- ^ "Greece Factsheet" (PDF). Global Cancer Observatory. Retrieved 9 November 2019.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 11 November 2009. Retrieved 11 November 2009.
- ^ Roth, Gregory A.; Abate, Degu; Abate, Kalkidan Hassen; Abay, Solomon M.; Abbafati, Cristiana; Abbasi, Nooshin; Abbastabar, Hedayat; Abd-Allah, Foad; Abdela, Jemal; Abdelalim, Ahmed; Abdollahpour, Ibrahim; Abdulkader, Rizwan Suliankatchi; Abebe, Haftom Temesgen; Abebe, Molla; Abebe, Zegeye; Abejie, Ayenew Negesse; Abera, Semaw F.; Abil, Olifan Zewdie; Abraha, Haftom Niguse; Abrham, Aklilu Roba; Abu-Raddad, Laith Jamal; Accrombessi, Manfred Mario Kokou; Acharya, Dilaram; Adamu, Abdu A.; Adebayo, Oladimeji M.; Adedoyin, Rufus Adesoji; Adekanmbi, Victor; Adetokunboh, Olatunji O.; Adhena, Beyene Meressa; et al. (November 2018). "Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017". Lancet. 392 (10159): 1736–1788. doi:10.1016/S0140-6736(18)32203-7. PMC 6227606. PMID 30496103.
- ^ Lakkis NA, Adib SM, Hamadeh GN, El-Jarrah RT, Osman MH (2018). "Bladder Cancer in Lebanon: Incidence and Comparison to Regional and Western Countries". Cancer Control. 25 (1): 1073274818789359. doi:10.1177/1073274818789359. PMC 6055109. PMID 30027755.
- ^ Jump up to: a b Hemelt M, Yamamoto H, Cheng KK, Zeegers MP (January 2009). "The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses". International Journal of Cancer. 124 (2): 412–9. doi:10.1002/ijc.23856. PMID 18792102. S2CID 28518800.
- ^ "Scientists Find One Reason Why Bladder Cancer Hits More Men". University of Rochester Medical Center. 20 April 2007. Archived from the original on 11 January 2009. Retrieved 20 April 2007.
- ^ Kim A, Kim MS, Ahn JH, Choi WS, Park HK, Kim HG, Paick SH (November 2019). "Clinical significance of 5-α reductase inhibitor and androgen deprivation therapy in bladder cancer incidence, recurrence, and survival: a meta-analysis and systemic review". The Aging Male. 23 (5): 971–978. doi:10.1080/13685538.2019.1646238. PMID 31724468. S2CID 208017350.
- ^ "Canada Fact Sheet" (PDF). Global Cancer Observatory. Retrieved 9 November 2019.
- ^ Jump up to: a b "Bladder cancer statistics - Canadian Cancer Society". www.cancer.ca. Retrieved 2 December 2019.
- ^ "China Fact Sheet" (PDF). Global Cancer Observatory. Retrieved 9 November 2019.
- ^ Pang C, Guan Y, Li H, Chen W, Zhu G (June 2016). "Urologic cancer in China". Japanese Journal of Clinical Oncology. 46 (6): 497–501. doi:10.1093/jjco/hyw034. PMID 27049022.
- ^ team, FPFIS (8 March 2017). "Epidemiology of bladder cancer in Europe". EU Science Hub - European Commission. Retrieved 2 December 2019.
- ^ "UK fact sheet" (PDF). Global Cancer Observatory. Retrieved 9 November 2019.
- ^ Ward EM, Sherman RL, Henley SJ, Jemal A, Siegel DA, Feuer EJ, et al. (December 2019). "Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20-49 Years". Journal of the National Cancer Institute. 111 (12): 1279–1297. doi:10.1093/jnci/djz106. PMC 6910179. PMID 31145458.
- ^ "Key Statistics for Bladder Cancer". www.cancer.org. Retrieved 28 November 2019.
- ^ "Common Cancer Sites - Cancer Stat Facts". SEER. Retrieved 28 November 2019.
External links[]
| Classification | |
|---|---|
| External resources |
|
- Bladder cancer at Curlie
- Clinically reviewed bladder cancer information for patients, from Cancer Research UK
- Cancer.Net: Bladder Cancer
- EORTC calculator for non-muscle invasive bladder cancer risk stratification
- Bladder cancer
- Infectious causes of cancer