Brine pool

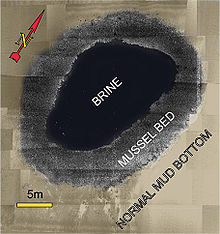

A brine pool, sometimes called an underwater, a deepwater lake, or a brine lake, is a volume of brine collected in a seafloor depression. The pools are dense bodies of water that have a salinity that is three to eight times greater than the surrounding ocean. Brine pools are commonly found below polar sea ice and in the deep ocean. Those below sea ice form through a process called brine rejection.[1] For deep-sea brine pools, salt is necessary to increase the salinity gradient. The salt can come from one of two processes: the dissolution of large salt deposits through salt tectonics[2] or geothermally heated brine issued from tectonic spreading centers.[3] The brine often contains high concentrations of hydrogen sulfide and methane, which provide energy to chemosynthetic organisms that live near the pool. These creatures are often extremophiles and symbionts.[4][5] Deep-sea and polar brine pools are toxic to marine animals due to their high salinity and anoxic properties,[1] which can ultimately lead to toxic shock and possibly death. The frequency of brine pool formation coupled with their uniquely high salinity has made them a candidate for research regarding ways to harness their properties to improve human science.[6]
Characteristics[]
Brine pools are sometimes called sea floor "lakes" because the dense brine does not easily mix with overlying seawater creating a distinct interface between water masses. The pools range in area from less than 1 square metre (11 sq ft) to as large as the 120 square kilometres (46 sq mi) Orca Basin.[2] The high salinity raises the density of the brine, which creates a surface and shoreline for the pool. Because of the brine's high density and lack of mixing currents in the deep ocean, brine pools often become anoxic and deadly to respiring organisms.[7] Brine pools supporting chemosynthetic activity, however, form life on the pool's shores where bacteria and their symbionts grow near the highest concentrations of nutrient release.[8] Patchy, reddish layers can be observed floating above the dense brine interface due high densities of halophilic archaea that are supported by these environments.[9] These shores are complex environments with significant shifts in salinity, oxygen concentration, pH, and temperature over a relatively small vertical scale. These transitions provide a variety of environmental niches.[10][11]
Formation[]
Brine pools are created through three primary methods: brine rejection below sea ice, dissolution of salts into bottom water through salt-tectonics, and geothermal heating of brine at tectonic boundaries and hot spots.
- Brine rejection
- When sea water freezes, salts do not fit into the crystalline structure of ice so the salts are expelled. The expelled salts form a cold, dense, brine that sinks below the sea ice to the sea floor. Brine rejection on an oceanic scale is associated with the formation of North Atlantic Deep Water (NADW) and Antarctic Bottom Water (AAW) that play a large role in global thermohaline circulation (THC). On a localized scale, that rejected brine collects in seafloor depressions forming a brine pool. In the absence of mixing, the brine will become anoxic in a matter of weeks.[1]
- Salt tectonics
- During the Middle Jurassic period, the Gulf of Mexico was a shallow sea that dried out, producing a thick layer of salt and seawater derived minerals up to 8 km thick. When the Gulf refilled with water, the salt layer was preserved from dissolution by sediments accumulating over the salt. Subsequent sedimentation layers became so heavy that they began to deform and move the more malleable salt layer below. In some places, the salt layer now protrudes at or near the seafloor where it can interact with seawater. Where seawater comes in contact with the salt, the deposits dissolve and form brines. The location of these surfacing Jurassic era salt deposits is also associated with methane releases giving deep ocean brine pools their chemical characteristics.[2]
- Geothermal heating
- At earth's oceanic tectonic spreading centers, plates are moving apart, allowing new magma to rise and cool. This process is involved in creating new sea floor. These mid-ocean ridges allow seawater to seep downward into fractures where they come in contact with and dissolve minerals. In the Red Sea for example, Red Sea Deep Water (RSDW) seeps into the fissures created at the tectonic boundary. The water dissolves salts from deposits created in the Miocene epoch much like the Jurassic period deposits in the Gulf of Mexico. The resulting brine is then superheated in the hydrothermal active zone over the magma chamber. The heated brine rises to the seafloor where it cools and settles in depressions as brine pools. The location of these pools is also associated with methane, hydrogen sulfide, and other chemical releases setting the stage for chemosynthetic activity.[3]
Support of life[]
Due to the methods of their formation and lack of mixing, brine pools are anoxic and deadly to most organisms. When an organism enters a brine pool, they attempt to "breathe" the environment and experience cerebral hypoxia due to the lack of oxygen and toxic shock from the hyper-salinity. If organisms cannot surface long enough to retreat to the rim, they quickly die.[12] When observed by submarines or Remotely Operated Vehicles (ROV), brine pools are found to be eerily littered with dead fish, crabs, amphipods, and various organisms that ventured too far into the brine. Dead organisms are then preserved in the brine for years without decay due to the anoxic nature of the pool preventing decay and creating a fish "graveyard."[8]
Despite the harsh conditions, life in the form of macrofauna such as bivalves can be found in a thin area along the rim of a brine pool. A novel genus and species of bivalves, known as Apachecorbula muriatica, have been found along the edge of the "Valdiva Deep" brine pool in the Red Sea.[13] There have also been recorded instances of macrofauna brine pools at the seawater interface. Inactive sulfur chimneys have been found with affiliated epifauna such as polychaetes and hydroids. In fauna like gastropods, capitellid polychaetes, and top snails have also been found to be associated with brine pools in the Red Sea. Such species typically feed on microbial symbionts or bacterial and detritus films.[14]
While organisms can typically flourish on the outskirts of a brine pool, they are not always safe from harm here. One possible reason for this is that underwater landslides can impact brine pools and cause waves of hypersaline brine to spill out into surrounding basins, thus negatively affecting the biological communities which live there.[15]
Despite their inhospitable nature, brine pools can also provide a home, allowing organisms to flourish. Deep-sea brine pools often coincide with cold seep activity allowing for chemosynthetic life to thrive. Methane and hydrogen sulfide released by the seep is processed by bacteria, which have a symbiotic relationship with organisms such as seep mussels.[16] The seep mussels create two distinct zones. The inner zone, which is at the edge of the pool, provides the best physiological conditions and allows for maximum growth. The outer zone is near the transition between the mussel bed and the surrounding seafloor, and this area provides the worst conditions causing these mussels to have lower maximum sizes and densities.[17] This ecosystem is dependent on chemical energy and, relative to almost all other life on Earth, has no dependence on energy from the Sun.[18]
An important part of the study of extreme environments such as brine pools is the function and survival of microbes. Microbes help support the larger biological community around environments like brine pools and are key to understanding the survival of other extremophiles. Biofilms contribute to the creation of microbes and are considered the foundation by which other micro-organisms can survive in extreme environments. The research into the growth and function of artificial extremophile biofilms has been slow due to the difficulty in recreating the extreme deep-sea environments they are found in.[19]
Examples[]
- Afifi[20]
- Atlantis II[21]
- Conrad[22]
- Discovery[23]
- Kebrit[24]
- Kryos [25]
- L'Atalante basin
- Orca Basin
- Shaban[26]
Future uses[]
One major idea involves harnessing the salinity of brine pools to use as a power source. This would be done using an osmotic engine which draws the high salinity top water through the engine and pushes it down due to osmotic pressure. This would cause the brackish stream (which is less dense and has a lighter salinity) to be propelled away from the engine via buoyancy. The energy created by this exchange can be harnessed using a turbine to create a power output.[7]
It is possible to study liquid brine in order to harness its electrical conductivity to study if liquid water is present on Mars.[6] A HABIT (Habitability: Brines, Irradiation, and Temperature) instrument will be part of a 2020 campaign to monitor changing conditions on Mars. This device will include a BOTTLE (Brine Observation Transition to Liquid Experiment) experiment to quantify the formation of transient liquid brine as well as observe its stability over time under non-equilibrium conditions.[6]
A third idea involves using microorganisms in deep-sea brine pools to form natural product drugs.[27] These microorganisms are important sources of bioactive molecules against various diseases due to the extreme environment they inhabit, giving potential to an increasing number of drugs in clinical trials.[28] In particular, a novel finding in a study used microorganisms from the Red Sea brine pools as potential anticancer drugs.[29][30][31]
Deep sea brine pools have also been a large interest in bioprospecting in the hope that unlikely environments might serve as sources of biomedical breakthroughs due to unexplored biodiversity. Some areas have been found to host antibacterial and anticancer activities in biosynthetic clusters.[32] Other novel antibiotic resistance enzymes have been found that are useful in various biomedical and industrial applications.[33]
References[]
- ^ Jump up to: a b c Kvitek, Rikk (February 1998). "Black pools of death: Hypoxic, brine-filled ice gouge depressions become lethal traps for benthic organisms in a shallow Arctic embayment". Marine Ecology Progress Series. 162: 1–10. Bibcode:1998MEPS..162....1K. doi:10.3354/meps162001 – via ResearchGate.
- ^ Jump up to: a b c "NOAA Ocean Explorer: Gulf of Mexico 2002". oceanexplorer.noaa.gov. Retrieved 2020-09-28.
- ^ Jump up to: a b Salem, Mohamed (2017-06-01). "Study of Conrad and Shaban deep brines, Red Sea, using bathymetric, parasound and seismic surveys". NRIAG Journal of Astronomy and Geophysics. 6 (1): 90–96. Bibcode:2017JAsGe...6...90S. doi:10.1016/j.nrjag.2017.04.003. S2CID 132353952.
- ^ Extremophile life near brine pools Archived November 10, 2006, at the Wayback Machine
- ^ Eder, W; Jahnke, LL; Schmidt, M; Huber, R (July 2001). "Microbial diversity of the brine-seawater interface of the Kebrit Deep, Red Sea, studied via 16S rRNA gene sequences and cultivation methods". Appl. Environ. Microbiol. 67 (7): 3077–85. doi:10.1128/AEM.67.7.3077-3085.2001. PMC 92984. PMID 11425725.
- ^ Jump up to: a b c Nazarious, Miracle Israel; Ramachandran, Abhilash Vakkada; Zorzano, Maria-Paz; Martin-Torres, Javier (2019-09-01). "Calibration and preliminary tests of the Brine Observation Transition To Liquid Experiment on HABIT/ExoMars 2020 for demonstration of liquid water stability on Mars". Acta Astronautica. 162: 497–510. Bibcode:2019AcAau.162..497N. doi:10.1016/j.actaastro.2019.06.026. ISSN 0094-5765.
- ^ Jump up to: a b Arias, Francisco J.; Heras, Salvador De Las (2019). "On the feasibility of ocean brine pool power stations". International Journal of Energy Research. 43 (15): 9049–9054. doi:10.1002/er.4708. hdl:2117/170786. ISSN 1099-114X.
- ^ Jump up to: a b "Brine Pools: The Underwater Lakes of Despair". www.amusingplanet.com. Retrieved 2020-09-28.
- ^ DasSarma, Shiladitya; DasSarma, Priya (2012-03-15), "Halophiles", eLS, Chichester, UK: John Wiley & Sons, Ltd, doi:10.1002/9780470015902.a0000394.pub3, ISBN 978-0-470-01617-6, retrieved 2020-11-02
- ^ Antunes, André; Olsson-Francis, Karen; McGenity, Terry J. (2020), "Exploring Deep-Sea Brines as Potential Terrestrial Analogues of Oceans in the Icy Moons of the Outer Solar System", Astrobiology: Current, Evolving, and Emerging Perspectives, Caister Academic Press, 38, pp. 123–162, doi:10.21775/9781912530304.06, ISBN 978-1-912530-30-4, PMID 31967579
- ^ Bougouffa, S.; Yang, J. K.; Lee, O. O.; Wang, Y.; Batang, Z.; Al-Suwailem, A.; Qian, P. Y. (June 2013). "Distinctive Microbial Community Structure in Highly Stratified Deep-Sea Brine Water Columns". Applied and Environmental Microbiology. 79 (11): 3425–3437. doi:10.1128/AEM.00254-13. ISSN 0099-2240. PMC 3648036. PMID 23542623.
- ^ Frazer, Jennifer. "Playing in a Deep-Sea Brine Pool Is Fun, as Long as You're an ROV [Video]". Scientific American Blog Network. Retrieved 2020-10-30.
- ^ Oliver, P. Graham; Vestheim, Hege; Antunes, André; Kaartvedt, Stein (May 2015). "Systematics, functional morphology and distribution of a bivalve ( Apachecorbula muriatica gen. et sp. nov.) from the rim of the 'Valdivia Deep' brine pool in the Red Sea". Journal of the Marine Biological Association of the United Kingdom. 95 (3): 523–535. doi:10.1017/S0025315414001234. hdl:1822/39421. ISSN 0025-3154. S2CID 55354847.
- ^ Vestheim, Hege; Kaartvedt, Stein (2015-02-26). "A deep sea community at the Kebrit brine pool in the Red Sea". Marine Biodiversity. 46 (1): 59–65. doi:10.1007/s12526-015-0321-0. ISSN 1867-1616. S2CID 16122787.
- ^ Sawyer, Derek E.; Mason, R. Alan; Cook, Ann E.; Portnov, Alexey (2019-01-15). "Submarine Landslides Induce Massive Waves in Subsea Brine Pools". Scientific Reports. 9 (1): 128. Bibcode:2019NatSR...9..128S. doi:10.1038/s41598-018-36781-7. ISSN 2045-2322. PMC 6333809. PMID 30644410. S2CID 58010364.
- ^ Hourdez, Stéphane; Frederick, Lee-Ann; Schernecke, Andrea; Fisher, Charles R. (2001). "Functional respiratory anatomy of a deep-sea orbiniid polychaete from the Brine Pool NR-1 in the Gulf of Mexico". Invertebrate Biology. 120 (1): 29–40. doi:10.1111/j.1744-7410.2001.tb00023.x. ISSN 1744-7410.
- ^ Smith, Emily B.; Scott, Kathleen M.; Nix, Erica R.; Korte, Carol; Fisher, Charles R. (September 2000). "Growth and Condition of Seep Mussels (Bathymodiolus Childressi) at a Gulf of Mexico Brine Pool". Ecology. 81 (9): 2392–2403. doi:10.1890/0012-9658(2000)081[2392:GACOSM]2.0.CO;2. ISSN 0012-9658.
- ^ World Wildlife Fund. "Deep sea ecology: hydrothermal vents and cold seeps." March 23, 2006. Accessed October 3, 2007.
- ^ Zhang, Weipeng; Wang, Yong; Bougouffa, Salim; Tian, Renmao; Cao, Huiluo; Li, Yongxin; Cai, Lin; Wong, Yue Him; Zhang, Gen; Zhou, Guowei; Zhang, Xixiang (2015). "Synchronized dynamics of bacterial niche-specific functions during biofilm development in a cold seep brine pool". Environmental Microbiology. 17 (10): 4089–4104. doi:10.1111/1462-2920.12978. hdl:10754/561085. ISSN 1462-2920. PMID 26171930.
- ^ Duarte, Carlos M.; Røstad, Anders; Michoud, Grégoire; Barozzi, Alan; Merlino, Giuseppe; Delgado-Huertas, Antonio; Hession, Brian C.; Mallon, Francis L.; Afifi, Abdulakader M.; Daffonchio, Daniele (2020-01-22). "Discovery of Afifi, the shallowest and southernmost brine pool reported in the Red Sea". Scientific Reports. 10 (1): 910. Bibcode:2020NatSR..10..910D. doi:10.1038/s41598-020-57416-w. ISSN 2045-2322. PMC 6976674. PMID 31969577. S2CID 210844928.
- ^ Wang, Yong; Yang, Jiang Ke; Lee, On On; Li, Tie Gang; Al-Suwailem, Abdulaziz; Danchin, Antoine; Qian, Pei-Yuan (2011-12-21). "Bacterial Niche-Specific Genome Expansion Is Coupled with Highly Frequent Gene Disruptions in Deep-Sea Sediments". PLOS ONE. 6 (12): e29149. Bibcode:2011PLoSO...629149W. doi:10.1371/journal.pone.0029149. ISSN 1932-6203. PMC 3244439. PMID 22216192.
- ^ Salem, Mohamed (2017-06-01). "Study of Conrad and Shaban deep brines, Red Sea, using bathymetric, parasound and seismic surveys". NRIAG Journal of Astronomy and Geophysics. 6 (1): 90–96. Bibcode:2017JAsGe...6...90S. doi:10.1016/j.nrjag.2017.04.003. ISSN 2090-9977. S2CID 132353952.
- ^ Siam, Rania; Mustafa, Ghada A.; Sharaf, Hazem; Moustafa, Ahmed; Ramadan, Adham R.; Antunes, Andre; Bajic, Vladimir B.; Stingl, Uli; Marsis, Nardine G. R.; Coolen, Marco J. L.; Sogin, Mitchell (2012-08-20). "Unique Prokaryotic Consortia in Geochemically Distinct Sediments from Red Sea Atlantis II and Discovery Deep Brine Pools". PLOS ONE. 7 (8): e42872. Bibcode:2012PLoSO...742872S. doi:10.1371/journal.pone.0042872. ISSN 1932-6203. PMC 3423430. PMID 22916172.
- ^ Abdallah, Rehab Z.; Adel, Mustafa; Ouf, Amged; Sayed, Ahmed; Ghazy, Mohamed A.; Alam, Intikhab; Essack, Magbubah; Lafi, Feras F.; Bajic, Vladimir B.; El-Dorry, Hamza; Siam, Rania (2014). "Aerobic methanotrophic communities at the Red Sea brine-seawater interface". Frontiers in Microbiology. 5: 487. doi:10.3389/fmicb.2014.00487. ISSN 1664-302X. PMC 4172156. PMID 25295031.
- ^ Yakimov, Michail M.; Cono, Violetta La; Spada, Gina L.; Bortoluzzi, Giovanni; Messina, Enzo; Smedile, Francesco; Arcadi, Erika; Borghini, Mireno; Ferrer, Manuel; Schmitt‐Kopplin, Phillippe; Hertkorn, Norbert (2015). "Microbial community of the deep-sea brine Lake Kryos seawater–brine interface is active below the chaotropicity limit of life as revealed by recovery of mRNA". Environmental Microbiology. 17 (2): 364–382. doi:10.1111/1462-2920.12587. ISSN 1462-2920. PMID 25622758.
- ^ Salem, Mohamed (2017-06-01). "Study of Conrad and Shaban deep brines, Red Sea, using bathymetric, parasound and seismic surveys". NRIAG Journal of Astronomy and Geophysics. 6 (1): 90–96. Bibcode:2017JAsGe...6...90S. doi:10.1016/j.nrjag.2017.04.003. ISSN 2090-9977. S2CID 132353952.
- ^ Li, Dehai; Wang, Fengping; Xiao, Xiang; Zeng, Xiang; Gu, Qian-Qun; Zhu, Weiming (May 2007). "A new cytotoxic phenazine derivative from a deep sea bacterium Bacillus sp". Archives of Pharmacal Research. 30 (5): 552–555. doi:10.1007/BF02977647. ISSN 0253-6269. PMID 17615672. S2CID 10515104.
- ^ Ziko, Laila; Saqr, Al-Hussein A.; Ouf, Amged; Gimpel, Matthias; Aziz, Ramy K.; Neubauer, Peter; Siam, Rania (2019-03-18). "Antibacterial and anticancer activities of orphan biosynthetic gene clusters from Atlantis II Red Sea brine pool". Microbial Cell Factories. 18 (1): 56. doi:10.1186/s12934-019-1103-3. ISSN 1475-2859. PMC 6423787. PMID 30885206.
- ^ Craig, H. (1966-12-23). "Isotopic Composition and Origin of the Red Sea and Salton Sea Geothermal Brines". Science. 154 (3756): 1544–1548. Bibcode:1966Sci...154.1544C. doi:10.1126/science.154.3756.1544. ISSN 0036-8075. PMID 17807292. S2CID 40574864.
- ^ Sagar, Sunil; Esau, Luke; Hikmawan, Tyas; Antunes, Andre; Holtermann, Karie; Stingl, Ulrich; Bajic, Vladimir B.; Kaur, Mandeep (2013-02-06). "Cytotoxic and apoptotic evaluations of marine bacteria isolated from brine-seawater interface of the Red Sea". BMC Complementary and Alternative Medicine. 13 (1): 29. doi:10.1186/1472-6882-13-29. ISSN 1472-6882. PMC 3598566. PMID 23388148.
- ^ Grötzinger, Stefan Wolfgang; Alam, Intikhab; Alawi, Wail Ba; Bajic, Vladimir B.; Stingl, Ulrich; Eppinger, Jörg (2014). "Mining a database of single amplified genomes from Red Sea brine pool extremophiles—improving reliability of gene function prediction using a profile and pattern matching algorithm (PPMA)". Frontiers in Microbiology. 5: 134. doi:10.3389/fmicb.2014.00134. ISSN 1664-302X. PMC 3985023. PMID 24778629.
- ^ Ziko, Laila; Saqr, Al-Hussein A.; Ouf, Amged; Gimpel, Matthias; Aziz, Ramy K.; Neubauer, Peter; Siam, Rania (2019-03-18). "Antibacterial and anticancer activities of orphan biosynthetic gene clusters from Atlantis II Red Sea brine pool". Microbial Cell Factories. 18 (1): 56. doi:10.1186/s12934-019-1103-3. ISSN 1475-2859. PMC 6423787. PMID 30885206.
- ^ Elbehery, Ali H. A.; Leak, David J.; Siam, Rania (2016-12-22). "Novel thermostable antibiotic resistance enzymes from the Atlantis II Deep Red Sea brine pool". Microbial Biotechnology. 10 (1): 189–202. doi:10.1111/1751-7915.12468. ISSN 1751-7915. PMC 5270753. PMID 28004885.
Further reading[]
- Boetius, A.; Joye, S. (2009-06-18). "Thriving in Salt". Science. 324 (5934): 1523–1525. doi:10.1126/science.1172979. ISSN 0036-8075.
- Eder, W., Jahnke, L. L., Schmidt, M., & Huber, R. (2001). Microbial Diversity of the Brine-Seawater Interface of the Kebrit Deep, Red Sea, Studied via 16S rRNA Gene Sequences and Cultivation Methods. Applied and Environmental Microbiology, 67(7), 3077-3085. doi:10.1128/aem.67.7.3077-3085.2001
- Guan, Y., Hikmawan, T., Antunes, A., Ngugi, D., & Stingl, U. (2015). Diversity of methanogens and sulfate-reducing bacteria in the interfaces of five deep-sea anoxic brines of the Red Sea. Research in Microbiology, 166(9), 688-699. doi:10.1016/j.resmic.2015.07.002
- Hartmann, M., Scholten, J., Stoffers, P., & Wehner, F. (1998). Hydrographic structure of brine-filled deeps in the Red Sea—new results from the Shaban, Kebrit, Atlantis II, and Discovery Deep. Marine Geology, 144(4), 311-330. doi:10.1016/s0025-3227(97)00055-8
- Patowary, K. (2018, November 07). Brine Pools: The Underwater Lakes of Despair. Retrieved October 28, 2020, from https://www.amusingplanet.com/2018/11/brine-pools-lakes-under-ocean.html
- US Department of Commerce, N. (n.d.). Gulf of Mexico 2002. Retrieved October 28, 2020, from https://oceanexplorer.noaa.gov/explorations/02mexico/welcome.html
- Wankel, S. D., Joye, S. B., Samarkin, V. A., Shah, S. R., Friederich, G., Melas-Kyriazi, J., & Girguis, P. R. (2010). New constraints on methane fluxes and rates of anaerobic methane oxidation in a Gulf of Mexico brine pool via in situ mass spectrometry. Deep Sea Research Part II: Topical Studies in Oceanography, 57(21-23), 2022-2029. doi:10.1016/j.dsr2.2010.05.009
- Bodies of water
- Coastal and oceanic landforms
