Carbazole

| |

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
9H-Carbazole[1] | |
| Other names
9-azafluorene
dibenzopyrrole diphenylenimine diphenyleneimide USAF EK-600 | |
| Identifiers | |
3D model (JSmol)
|
|
| 3956 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.542 |
| EC Number |
|
| 102490 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C12H9N | |
| Molar mass | 167.211 g·mol−1 |
| Density | 1.301 g cm−3 |
| Melting point | 246.3 °C (475.3 °F; 519.5 K)[2] |
| Boiling point | 354.69 °C (670.44 °F; 627.84 K)[2] |
| −117.4 × 10−6 cm3 mol−1 | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
GHS hazard statements
|
H341, H351, H400, H411, H413 |
| P201, P202, P273, P281, P308+313, P391, P405, P501 | |
| Flash point | 220 °C (428 °F; 493 K) [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole (equivalent to the 9a–4a double bond in carbazole, respectively).
Carbazole is a constituent of tobacco smoke.[3]
Synthesis[]
A classic laboratory organic synthesis for carbazole is the Borsche–Drechsel cyclization.[4][5]
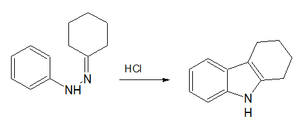
In the first step, phenylhydrazine is condensed with cyclohexanone to the corresponding imine. The second step is a hydrochloric acid-catalyzed rearrangement reaction and ring-closing reaction to . In one modification, both steps are rolled into one by carrying out the reaction in acetic acid.[6] In the third step, this compound is oxidized by red lead to carbazole itself.
Another classic is the Bucherer carbazole synthesis, which uses a naphthol and an aryl hydrazine.[7]

A third method for the synthesis of carbazole is the Graebe–Ullmann reaction.

In the first step, an N-phenyl-1,2-diaminobenzene (N-phenyl-o-phenylenediamine) is converted into a diazonium salt which instantaneously forms a 1,2,3-triazole. The triazole is unstable and at elevated temperatures, nitrogen is released and the carbazole is formed.[8][9]
Applications[]
Aminoethylcarbazole is used in the production of pigment violet 23.

- Rimcazole is also made from carbazole proper.
- Carprofen is another use.
- & are both uncommon, but still technical case examples.
Related aromatic compounds[]
- Indoline
- Indole
- Carboline
- Fluorene
- Pyrrole
- N-Vinylcarbazole
References[]
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 212. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Jump up to: a b c Lide, David R. (2007). CRC Handbook of Chemistry and Physics, 88th Edition. CRC Press. pp. 3–86. ISBN 978-0-8493-0488-0.
- ^ Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). "Hazardous Compounds in Tobacco Smoke". Int. J. Environ. Res. Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. PMC 3084482. PMID 21556207.
- ^ W. Borsche (1908). "Ueber Tetra- und Hexahydrocarbazolverbindungen und eine neue Carbazolsynthese. (Mitbearbeitet von. A. Witte und W. Bothe.)". Justus Liebigs Ann. Chem. (in German). 359 (1–2): 49–80. doi:10.1002/jlac.19083590103.
- ^ E. Drechsel (1888). "Ueber Elektrolyse des Phenols mit Wechselströmen". J. Prakt. Chem. (in German). 38 (1): 65–74. doi:10.1002/prac.18880380105.
- ^ Rogers, Crosby U.; Corson, B. B. (1950). "1,2,3,4-Tetrahydrocarbazole (Carbazole, 1,2,3,4-tetrahydro-)". Organic Syntheses. 30: 90. doi:10.15227/orgsyn.030.0090.; Collective Volume, 4, p. 884
- ^ Wang, Zerong (2010). "Bucherer Carbazole Synthesis". Comprehensive Organic Name Reactions and Reagents. doi:10.1002/9780470638859.conrr120. ISBN 9780470638859.
- ^ Carl Graebe; Fritz Ullmann (1896). "Ueber eine neue Carbazolsynthese". Justus Liebigs Ann. Chem. (in German). 291 (1): 16–17. doi:10.1002/jlac.18962910104.
- ^ O. Bremer (1934). "Über die Bedeutung der Graebe-Ullmannschen Carbazolsynthese und deren Übertragung auf N-substituierte Pyridino-triazole". Justus Liebigs Ann. Chem. (in German). 514 (1): 279–291. doi:10.1002/jlac.19345140116.
External links[]
| Wikisource has the text of the 1911 Encyclopædia Britannica article "Carbazol". |
- Carbazoles
- IARC Group 2B carcinogens
