Imine

An imine (/ɪˈmiːn/ or /ˈɪmɪn/) is a functional group or chemical compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). If this group is not a hydrogen atom, then the compound can sometimes be referred to as a Schiff base.[1] The carbon atom has two additional single bonds.[2][3][4] The term "imine" was coined in 1883 by the German chemist Albert Ladenburg.[5]
Nomenclature and classification[]
Usually imines refer to compounds with the connectivity R2C=NR, as discussed below. In the older literature, imine refers to the aza analogue of an epoxide. Thus, ethyleneimine is the three-membered ring species C2H4NH.[6] The relationship of imines to amines having double and single bonds carries over to imides and amides, as in acetamide vs succinimide.
Imines are related to ketones and aldehydes by replacement of the oxygen with an NR group. When R = H, the compound is a primary imine, when R is hydrocarbyl, the compound is a secondary imine. Imines exhibit diverse reactivity and are commonly encountered throughout chemistry.[4] When R3 is OH, the imine is called an oxime, and when R3 is NH2 the imine is called a hydrazone.
A primary imine in which C is attached to both a hydrocarbyl and a H is called a primary aldimine; a secondary imine with such groups is called a secondary aldimine.[7] A primary imine in which C is attached to two hydrocarbyls is called a primary ketimine; a secondary imine with such groups is called a secondary ketimine.[8]

Primary aldimine
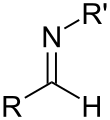
Secondary aldimine

Primary ketimine

Secondary ketimine

Aziridine and its derivatives are sometimes referred to as imines.
One way of naming aldimines is to take the name of the radical, remove final "e", and add "-imine", for example ethanimine. Alternately, an imine is named as a derivative of a carbonyl, adding the word "imine" to the name of a carbonyl compound whose oxo group is replaced by an imino group, for example sydnone imine and acetone imine (an intermediate in the synthesis of acetone azine).
N-Sulfinyl imines are a special class of imines having a sulfinyl group attached to the nitrogen atom.
An iminium cation is a related functional group in which the nitrogen has a fourth bond, giving it a positive charge.
Aldimine[]
An aldimine is an imine that is an analog of an aldehyde.[9] As such, aldimines have the general formula R–CH=N–R'. Aldimines are similar to ketimines, which are analogs of ketones.
An important subset of aldimines are the Schiff bases, in which the substituent on the nitrogen atom (R') is an alkyl or aryl group (i.e. not a hydrogen atom).[10]
| Nomenclature | CH3–CH2–CH2–CH=NH | CH3–CH=N–CH3 |
|---|---|---|
| 1 | ||
| 2 | butylideneazane | ethylidene(methyl)azane |
| 3 | butylideneamine | N-methylethylideneamine |
| common usage | butyraldehyde imine | acetaldehyde N-methylimine |
Aldimines may be named in three different manners:[11]
- by replacing the final -e of the parent hydride, R–CH3, with the suffix "-imine";
- as alkylidene derivatives of azane;
- (rare) as alkylidene derivatives of "amine".
An obsolete nomenclature treats aldimines as derivatives of a parent aldehyde.
Synthesis of imines[]

Imines are typically prepared by the condensation of primary amines and aldehydes.[12][13] Ketones undergo similar reactions, but less commonly than aldehydes. In terms of mechanism, such reactions proceed via the nucleophilic addition giving a hemiaminal -C(OH)(NHR)- intermediate, followed by an elimination of water to yield the imine (see alkylimino-de-oxo-bisubstitution for a detailed mechanism). The equilibrium in this reaction usually favors the carbonyl compound and amine, so that azeotropic distillation or use of a dehydrating agent, such as molecular sieves or magnesium sulfate, is required to push the reaction in favor of imine formation. In recent years, several reagents such as Tris(2,2,2-trifluoroethyl)borate [B(OCH2CF3)3],[14] pyrrolidine[15] or titanium ethoxide [Ti(OEt)4][16] have been shown to catalyse imine formation.
More specialized methods[]
Several other methods exist for the synthesis of imines.
- Reaction of organic azides with metal carbenoids (produced from diazocarbonyl compounds).[17]
- Condensation of carbon acids with nitroso compounds.
- The rearrangement of trityl N-haloamines in the Stieglitz rearrangement.
- Dehydration of hemiaminals.[18]
- By reaction of alkenes with hydrazoic acid in the Schmidt reaction.
- By reaction of a nitrile, hydrochloric acid, and an arene in the Hoesch reaction.
- Multicomponent synthesis of 3-thiazolines in the Asinger reaction.
- Primary ketimines can be synthesized via a Grignard reaction with a nitrile.[19][20]
Reactions[]

Imines are susceptible to hydrolysis to the corresponding amine and carbonyl compound. An imine reacts with an amine to an aminal, see for example the synthesis of cucurbituril.
Precursors to heterocycles[]
An imine reacts with dienes in the Imine Diels-Alder reaction to a tetrahydropyridine.
Imines are widely used as intermediates in the synthesis of heterocycles. Aromatic imines reacts with an enol ether to a quinoline in the Povarov reaction. The C=N bond in imines is reactive toward cycloadditions. Imines react, thermally, with ketenes in [2+2] cycloadditions to form β-lactams in the Staudinger synthesis. An imine can be oxidized with meta-chloroperoxybenzoic acid (mCPBA) to give an oxaziridine
A tosylimine reacts with an α,β-unsaturated carbonyl compound to an allylic amine in the Aza-Baylis–Hillman reaction.
Imines are intermediates in the alkylation of amines with formic acid in the Eschweiler-Clarke reaction.
A rearrangement in carbohydrate chemistry involving an imine is the Amadori rearrangement.
A methylene transfer reaction of an imine by an unstabilised sulphonium ylide can give an aziridine system. Imine react with dialkylphosphite in the Pudovik reaction and Kabachnik–Fields reaction
Acid-base reactions[]
Somewhat like the parent amines, imines are mildly basic and reversibly protonate to give iminium salts. Iminium derivatives are particularly susceptible to reduction to the amines using transfer hydrogenation or by the stoichiometric action of sodium cyanoborohydride. Since imines derived from unsymmetrical ketones are prochiral, their reduction is a useful method for the synthesis of chiral amines.
Imines are common ligands in coordination chemistry. The condensation of salicylaldehyde and ethylenediamine give families of imine-containing chelating agents such as salen.
Imine reductions[]
Imines are reduced via reductive amination. An imine can be reduced to an amine via hydrogenation for example in a synthesis of m-tolylbenzylamine:[21]
Other reducing agents are lithium aluminium hydride and sodium borohydride.[22]
The first asymmetric imine reduction was reported in 1973 by Kagan using Ph(Me)C=NBn and PhSiH2 in a hydrosilylation with chiral ligand DIOP and rhodium catalyst (RhCl(CH2CH2)2)2.[23] Many systems have since been investigated.[24][25]
Polymerisation[]
Unhindered aldimines tend to cyclize, as illustrated by the condensation of methylamine and formaldehyde, which gives the hexahydro-1,3,5-triazine.
Imine polymers (polyimines) can be synthesised from multivalent aldehydes and amines.[26] This polymerisation reaction generally proceeds upon mixing the relevant components.[27] Polyimines are particularly interesting materials because of their application as vitrimers. They are known to be easily recyclable and self-healing.[28]
Biological role[]
Imines are fairly common in nature.[29][30]
See also[]
- Enamine
- Schiff base
- Carboximidate
- Oxime
- Oxazolidine
- Other functional groups with a CN double bond: oximes, hydrazones
- Other functional groups with a CN triple bond: nitriles, isonitriles
References[]
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Schiff base". doi:10.1351/goldbook.S05498
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "imines". doi:10.1351/goldbook.I02957
- ^ March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. ISBN 0-471-85472-7
- ^ a b "Amines and Imines". Nomenclature of Organic Compounds. Advances in Chemistry. Vol. 126. 1974. pp. 180–188. doi:10.1021/ba-1974-0126.ch023. ISBN 9780841201910.
- ^ Ladenburg, A. (1883) "Ueber die Imine" (About imines), Berichte der Deutschen Chemischen Gesellschaft, 16 : 1149–1152 ; see p. 1150. From p. 1150: "Denn offenbar gehört auch das Piperidin in die Klasse der von mir gesuchten Verbindungen, für welche der Name Imine durch die bestehende Nomenklatur angezeigt ist." (For obviously piperidine also belongs in the class of compounds that are sought by me, for which the name "imines" is indicated by the prevailing nomenclature.)
- ^ "Ethylenimine". Organic Syntheses. 30: 38. 1950. doi:10.15227/orgsyn.030.0038.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "aldimines". doi:10.1351/goldbook.A00209.html
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "ketimines". doi:10.1351/goldbook.K03381.html
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "aldimines". doi:10.1351/goldbook.A00209
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Schiff bases (Schiff's bases)". doi:10.1351/goldbook.S05498
- ^ Panico R, Powell WH, Richer JC, eds. (1993). "Recommendation R-5.4.3". A Guide to IUPAC Nomenclature of Organic Compounds. IUPAC/Blackwell Science. pp. 89–90. ISBN 0-632-03488-2.
- ^ G. Wittig, A. Hesse (1970). "Directed Aldol Condensations:b-Phenylcinnamaldehyde". Organic Syntheses. 50: 66. doi:10.15227/orgsyn.050.0066.
- ^ Lucius A. Bigelow and Harry Eatough (1928). "Benzalaniline". Organic Syntheses. 8: 22. doi:10.15227/orgsyn.008.0022.
- ^ Reeves, Jonathan T.; Visco, Michael D.; Marsini, Maurice A.; Grinberg, Nelu; Busacca, Carl A.; Mattson, Anita E.; Senanayake, Chris H. (2015-05-15). "A General Method for Imine Formation Using B(OCH2CF3)3". Organic Letters. 17 (10): 2442–2445. doi:10.1021/acs.orglett.5b00949. ISSN 1523-7060. PMID 25906082.
- ^ Morales, Sara; Guijarro, Fernando G.; García Ruano, José Luis; Cid, M. Belén (2014-01-22). "A General Aminocatalytic Method for the Synthesis of Aldimines". Journal of the American Chemical Society. 136 (3): 1082–1089. doi:10.1021/ja4111418. ISSN 0002-7863. PMID 24359453.
- ^ Collados, Juan F.; Toledano, Estefanía; Guijarro, David; Yus, Miguel (2012-07-06). "Microwave-Assisted Solvent-Free Synthesis of Enantiomerically Pure N-(tert-Butylsulfinyl)imines". The Journal of Organic Chemistry. 77 (13): 5744–5750. doi:10.1021/jo300919x. ISSN 0022-3263. PMID 22694241.
- ^ Mandler, Michael; Truong, Phong; Zavalij, Peter; Doyle, Michael (Jan 14, 2014). "Catalytic Conversion of Diazocarbonyl Compounds to Imines: Applications to the Synthesis of Tetrahydropyrimidines and β-Lactams". Organic Letters. 16 (3): 740–743. doi:10.1021/ol403427s. PMID 24423056.
- ^ a b Middleton, W. J.; Carlson, H. D. (1970). "Hexafluoroacetone imine". Org. Syntheses. 50: 81–3. doi:10.15227/orgsyn.050.0081.
- ^ Koos, Miroslav; Mosher, Harry S. (1993). "α-Amino-α-trifluoromethyl-phenylacetonitrile: A potential reagent for NMR determination of enantiomeric purity of acids". Tetrahedron. 49 (8): 1541–1546. doi:10.1016/S0040-4020(01)80341-0.
- ^ Moureu, Charles; Mignonac, Georges (1920). "Les Cetimines". Annales de Chimie. 9 (13): 322–359. Retrieved 18 June 2014.
- ^ C. F. H. Allen and James VanAllan (1955). "m-Tolylbenzylamine". Organic Syntheses: 827.; Collective Volume, vol. 3
- ^ For example: Ieva R. Politzer and A. I. Meyers (1988). "Aldehydes from 2-Benzyl-4,4,6-trimethyl-5,6-dihydro-1,3(4H)-oxazine: 1-Phenylcyclopentanecarboxaldehyde". Organic Syntheses.; Collective Volume, vol. 6, p. 905
- ^ Langlois, N (1973). "Synthese asymetrique d'amines par hydrosilylation d'imines catalysee par un complexe chiral du rhodium". Tetrahedron Lett. 14 (49): 4865–4868. doi:10.1016/S0040-4039(01)87358-5.
- ^ Kobayashi, Shū; Ishitani, Haruro (1999). "Catalytic Enantioselective Addition to Imines". Chem. Rev. 99 (5): 1069–94. doi:10.1021/cr980414z. PMID 11749440.
- ^ J. Martens: Reduction of Imino Groups (C=N) in (G. Helmchen, R. W. Hoffmann, J. Mulzer, E. Schaumann) Houben-Weyl Stereoselective Synthesis, Workbench Edition E21 Volume 7, S. 4199-4238, Thieme Verlag Stuttgart, 1996, ISBN 3-13-106124-3.
- ^ Schoustra, Sybren K.; Groeneveld, Timo; Smulders, Maarten M. J. (2021). "The effect of polarity on the molecular exchange dynamics in imine-based covalent adaptable networks". Polymer Chemistry. 12 (11): 1635–1642. doi:10.1039/D0PY01555E.
- ^ Schoustra, Sybren K.; Dijksman, Joshua A.; Zuilhof, Han; Smulders, Maarten M. J. (2021). "Molecular control over vitrimer-like mechanics – tuneable dynamic motifs based on the Hammett equation in polyimine materials". Chemical Science. 12 (1): 293–302. doi:10.1039/d0sc05458e. ISSN 2041-6520. PMC 8178953. PMID 34163597.
- ^ Zhu, Jiaqi (2020). "A self-healing transparent polydimethylsiloxane elastomer based on imine bonds". European Polymer Journal. 123: 109382. doi:10.1016/j.eurpolymj.2019.109382. S2CID 214199868.
- ^ "Researchers look to nature to unearth the secrets of cyclic imine cleavage". EurekAlert!. Retrieved 2021-07-22.
- ^ Borchert, Andrew J.; Ernst, Dustin C.; Downs, Diana M. (2019). "Reactive enamines and imines in vivo: Lessons from the RidA paradigm". Trends in Biochemical Sciences. 44 (10): 849–860. doi:10.1016/j.tibs.2019.04.011. ISSN 0968-0004. PMC 6760865. PMID 31103411.
- Imines
- Functional groups





