Allyl group

An allyl group is a substituent with the structural formula H2C=CH−CH2R, where R is the rest of the molecule. It consists of a methylene bridge (−CH2−) attached to a vinyl group (−CH=CH2).[1][2] The name is derived from the Latin word for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl".[3][4] The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.
Nomenclature[]
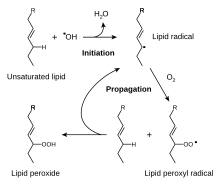
A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, CH2=CHCH2OH "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. This heightened reactivity has many practical consequences. The industrial production of acrylonitrile by ammoxidation of propene exploits the easy oxidation of the allylic C−H centers:
Unsaturated fats spoil by rancidification involving attack at allylic C−H centers.
Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reactions that tend to occur with allylic compounds are allylic oxidations, ene reactions, and the Tsuji–Trost reaction. Benzylic groups are related to allyl groups; both show enhanced reactivity.
Pentadienyl[]
A CH2 group connected to two vinyl groups is said to be doubly allylic. The bond dissociation energy of C−H bonds on a doubly allylic centre is about 10% less than the bond dissociation energy of a C−H bond that is allylic. The weakened C−H bonds reflect the high stability of the resulting pentadienyl radicals. Compounds containing the C=C−CH2−C=C linkages, e.g. linoleic acid derivatives, are prone to autoxidation, which can lead to polymerization or form semisolids. This reactivity pattern is fundamental to the film-forming behavior of the "drying oils", which are components of oil paints and varnishes.
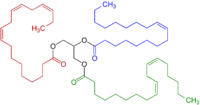
Homoallylic[]
The term homoallylic refers to the position on a carbon skeleton next to an allylic position. In but-3-enyl chloride CH2=CHCH2CH2Cl, the chloride is homoallylic because it is bonded to the homoallylic site.
Bonding[]
The allyl group is widely encountered in organic chemistry.[1] Allylic radicals, anions, and cations are often discussed as intermediates in reactions. All feature three contiguous sp²-hybridized carbon centers and all derive stability from resonance.[5] Each species can be presented by two resonance structures with the charge or unpaired electron distributed at both 1,3 positions.
 Resonance structure of the allyl anion
Resonance structure of the allyl anion
In terms of MO theory, the MO diagram has three molecular orbitals: the first one bonding, the second one non-bonding and the higher energy orbital is antibonding.[2]
 MO diagram for π orbitals on the allyl radical. The middle MO labeled Ψ2 is singly occupied in the allyl radical. In the allyl cation Ψ2 is unoccupied and in the allyl anion it is doubly occupied. Hydrogen atoms are omitted from this picture.
MO diagram for π orbitals on the allyl radical. The middle MO labeled Ψ2 is singly occupied in the allyl radical. In the allyl cation Ψ2 is unoccupied and in the allyl anion it is doubly occupied. Hydrogen atoms are omitted from this picture.
Reactions[]
Carbonyl allylation[]
Carbonyl allylation is one of the most common methods used to introduce an allyl group. Carbonyl allylation refers to the addition of an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol, as illustrated by the following two-step process. The first carbonyl allylation was reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.[6]
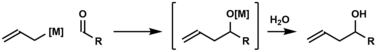
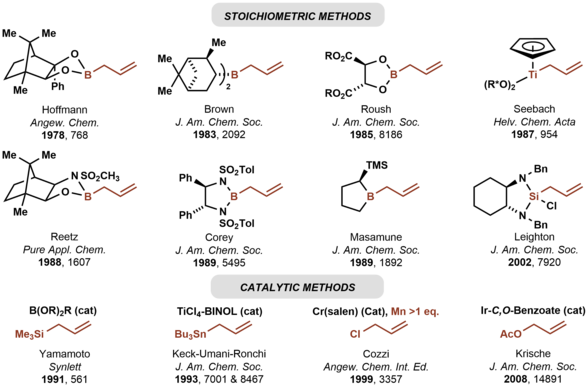
The first chiral allylmetal reagents for enantioselective carbonyl allylation were developed by Hoffmann.[7] Many chiral allylmetal reagents were subsequently developed.[8][9][10][11][12][13] Among them, the "Brown reagent", allyldiisopinocampheylborane, has found broad use.[9] As illustrated by the Keck allylation,[14] catalytic enantioselective additions of achiral allylmetal reagents to carbonyl compounds also are possible.[15] Whereas the aforementioned asymmetric carbonyl allylations rely on preformed allylmetal reagents, the Krische allylation exploits allyl acetate, an abundant chemical feedstock, for enantioselective carbonyl allylation.[16] Selected methods for asymmetric carbonyl allylation are summarized below.
Carbonyl allylation is often used to construct polyketide natural products and other oxygenated molecules with a contiguous array of stereocenters. For example, Steven V. Ley and coworkers used an allylstannanation of a threose-derived aldehyde to construct the macrolide antascomicin B, which structurally resembles FK506 and rapamycin, and is a potent binder of FKBP12.[17] The Krische allylation was used to prepare the polyketide natural product (+)-SCH 351448, which is a macrodiolide ionophore bearing 14 stereogenic centers.[18]

Conjugate addition[]
Conjugate allylation refers to the addition of an allyl group to the beta-position of an enone. The Hosomi-Sakurai reaction is the most commonly utilized method for conjugate allylation.[19]
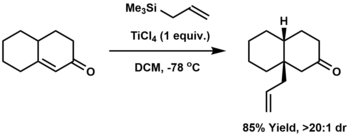
Allyl compounds[]
Many substituents can be attached to the allyl group to give stable compounds. Commercially important allyl compounds include:
- Allyl alcohol (H2C=CH−CH2OH)
- Allyl chloride (H2C=CH−CH2Cl)
- Crotyl alcohol (CH3CH=CH−CH2OH)
- Dimethylallyl pyrophosphate, central in the biosynthesis of terpenes, a precursor to many natural products, including natural rubber.
- Transition-metal allyl complexes, such as allylpalladium chloride dimer
See also[]
| Wikiquote has quotations related to: Allyl group |
- Allylic strain
- Carroll rearrangement
- Allylic palladium complex
- Tsuji–Trost reaction
- Propargylic/Homopropargylic
- Benzylic
- Vinylic
- Acetylenic
- Naloxone
- Allylic rearrangement
- Diallyllysergamide
- Terpene
References[]
- ^ Jump up to: a b Jerry March, "Advanced Organic Chemistry" 4th Ed. J. Wiley and Sons, 1992: New York. ISBN 0-471-60180-2.
- ^ Jump up to: a b Organic Chemistry 4th Ed. Morisson & Boyd 1988.
- ^ Theodor Wertheim (1844). "Untersuchung des Knoblauchöls". Annalen der Chemie und Pharmacie. 51 (3): 289–315. doi:10.1002/jlac.18440510302.
- ^ Eric Block (2010). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. ISBN 978-0-85404-190-9.
- ^ Organic Chemistry John McMurry 2nd ed. 1988
- ^ Michael; Saytzeff, Alexander (1877). "Synthese des Allyldimethylcarbinols". Justus Liebigs Annalen der Chemie. 185 (2–3): 151–169. doi:10.1002/jlac.18771850204. ISSN 1099-0690.
- ^ Herold, Thomas; Hoffmann, Reinhard W. (October 1978). "Enantioselective Synthesis of Homoallyl Alcoholsvia Chiral Allylboronic Esters". Angewandte Chemie International Edition in English. 17 (10): 768–769. doi:10.1002/anie.197807682. ISSN 0570-0833.
- ^ Hayashi, Tamio; Konishi, Mitsuo; Kumada, Makoto (September 1982). "Optically active allylsilanes. 2. High stereoselectivity in asymmetric reaction with aldehydes producing homoallylic alcohols". Journal of the American Chemical Society. 104 (18): 4963–4965. doi:10.1021/ja00382a046. ISSN 0002-7863.
- ^ Jump up to: a b Brown, Herbert C.; Jadhav, Prabhakar K. (April 1983). "Asymmetric carbon-carbon bond formation via .beta.-allyldiisopinocampheylborane. Simple synthesis of secondary homoallylic alcohols with excellent enantiomeric purities". Journal of the American Chemical Society. 105 (7): 2092–2093. doi:10.1021/ja00345a085. ISSN 0002-7863.
- ^ Roush, William R.; Walts, Alan E.; Hoong, Lee K. (December 1985). "Diastereo- and enantioselective aldehyde addition reactions of 2-allyl-1,3,2-dioxaborolane-4,5-dicarboxylic esters, a useful class of tartrate ester modified allylboronates". Journal of the American Chemical Society. 107 (26): 8186–8190. doi:10.1021/ja00312a062. ISSN 0002-7863.
- ^ Kinnaird, James W. A.; Ng, Pui Yee; Kubota, Katsumi; Wang, Xiaolun; Leighton, James L. (2002-07-01). "Strained Silacycles in Organic Synthesis: A New Reagent for the Enantioselective Allylation of Aldehydes". Journal of the American Chemical Society. 124 (27): 7920–7921. doi:10.1021/ja0264908. ISSN 0002-7863.
- ^ Short, Robert P.; Masamune, Satoru (March 1989). "Asymmetric allylboration with B-allyl-2-(trimethylsilyl)borolane". Journal of the American Chemical Society. 111 (5): 1892–1894. doi:10.1021/ja00187a061. ISSN 0002-7863.
- ^ Corey, E. J.; Yu, Chan Mo; Kim, Sung Soo (July 1989). "A practical and efficient method for enantioselective allylation of aldehydes". Journal of the American Chemical Society. 111 (14): 5495–5496. doi:10.1021/ja00196a082. ISSN 0002-7863.
- ^ Keck, Gary E.; Tarbet, Kenneth H.; Geraci, Leo S. (September 1993). "Catalytic asymmetric allylation of aldehydes". Journal of the American Chemical Society. 115 (18): 8467–8468. doi:10.1021/ja00071a074. ISSN 0002-7863.
- ^ Denmark, Scott E.; Fu, Jiping (2003-08-01). "Catalytic Enantioselective Addition of Allylic Organometallic Reagents to Aldehydes and Ketones". Chemical Reviews. 103 (8): 2763–2794. doi:10.1021/cr020050h. ISSN 0009-2665.
- ^ Kim, In Su; Ngai, Ming-Yu; Krische, Michael J. (2008-11-05). "Enantioselective Iridium-Catalyzed Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level via Transfer Hydrogenative Coupling of Allyl Acetate: Departure from Chirally Modified Allyl Metal Reagents in Carbonyl Addition". Journal of the American Chemical Society. 130 (44): 14891–14899. doi:10.1021/ja805722e. ISSN 0002-7863. PMC 2890235. PMID 18841896.
- ^ Brittain, Dominic E. A.; Griffiths-Jones, Charlotte M.; Linder, Michael R.; Smith, Martin D.; McCusker, Catherine; Barlow, Jaqueline S.; Akiyama, Ryo; Yasuda, Kosuke; Ley, Steven V. (2005). "Total Synthesis of Antascomicin B". Angewandte Chemie International Edition. 44 (18): 2732–2737. doi:10.1002/anie.200500174. ISSN 1521-3773.
- ^ Wang, Gang; Krische, Michael J. (2016-07-06). "Total Synthesis of (+)-SCH 351448: Efficiency via Chemoselectivity and Redox-Economy Powered by Metal Catalysis". Journal of the American Chemical Society. 138 (26): 8088–8091. doi:10.1021/jacs.6b04917. ISSN 0002-7863. PMC 4935581. PMID 27337561.
- ^ Hosomi, Akira; Sakurai, Hideki (March 1977). "Chemistry of organosilicon compounds. 99. Conjugate addition of allylsilanes to .alpha.,.beta.-enones. A New method of stereoselective introduction of the angular allyl group in fused cyclic .alpha.,.beta.-enones". Journal of the American Chemical Society. 99 (5): 1673–1675. doi:10.1021/ja00447a080. ISSN 0002-7863.
- Alkenyl groups
- Allyl compounds

