Amide (functional group)
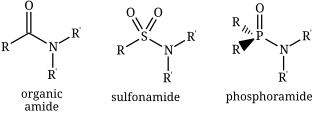
Structures of three kinds of amides: an organic amide (carboxamide), a sulfonamide, and a phosphoramide.
In chemistry, the term amide (/ˈæmaɪd/ or /ˈæmɪd/ or /ˈeɪmaɪd/)[1][2][3] is a compound with the functional group RnE(=O)xNR2, where n and x may be 1 or 2, E is some element, and each R represents an organic group or hydrogen.[4] It is a derivative of an oxoacid RnE(=O)xOH with an hydroxy group –OH replaced by an amine group –NR2.
Some important subclasses are
- carboxamides, or organic amides, where E = carbon, with the general formula RC(=O)NR2.
- phosphoramides, where E = phosphorus, such as R2P(=O)NR2
- sulfonamides, where E = sulfur, namely RS(=O)2NR2
The term amide may also refer to
- amide group, a functional group –C(=O)N= consisting of a carbonyl adjacent to a nitrogen atom.
- cyclic amide or lactam, a cyclic compound with the amide group –C(=O)N– in the ring.
- metal amide, an ionic compound ("salt") with the azanide anion H2N− (the conjugate base of ammonia) or to a derivative thereof R2N−.
There is also a neutral amino radical (•NH2) and a positively charged NH2+ ion called a nitrenium ion, but both of these are very unstable.
See also[]
References[]
- ^ "Amide definition and meaning - Collins English Dictionary". www.collinsdictionary.com. Retrieved 15 April 2018.
- ^ "amide". The American Heritage Dictionary of the English Language (5th ed.). Boston: Houghton Mifflin Harcourt.
- ^ "amide - Definition of amide in English by Oxford Dictionaries". Oxford Dictionaries - English. Retrieved 15 April 2018.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "amides". doi:10.1351/goldbook.A00266
Categories:
- Amides