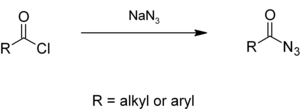Azide
It has been suggested that this article be split into a new article titled . (Discuss) (September 2021) |

Azide is the anion with the formula N−
3. It is the conjugate base of hydrazoic acid (HN3). N−
3 is a linear anion that is isoelectronic with CO2, NCO−, N2O, NO+
2 and NCF. Per valence bond theory, azide can be described by several resonance structures; an important one being . Azide is also a functional group in organic chemistry, RN3.[1]
The dominant application of azides is as a propellant in air bags.
History[]
Phenyl azide ("diazoamidobenzol"), was prepared in 1864 by Peter Griess by the reaction of ammonia and phenyldiazonium.[2][3] In the 1890s, Theodor Curtius, who had discovered hydrazoic acid (HN3), described the rearrangement of acyl azides to isocyanates subsequently named the Curtius rearrangement.[4] Rolf Huisgen described the eponymous 1,3-dipolar cycloaddition.[5][6]
The interest in azides among organic chemists has been relatively modest due to the reported instability of these compounds.[7] The situation has changed dramatically with the discovery by Sharpless et al. of Cu-catalysed (3+2)-cycloadditions between organic azides and terminal alkynes.[8][9] The azido- and the alkyne groups are "bioorthogonal", which means they do not interact with living systems, and at the same time they undergo an impressively fast and selective coupling. This type of formal 1,3-dipolar cycloaddition became the most famous example of so-called "click chemistry"[10][11] (perhaps, the only one known to a non-specialist), and the field of organic azides exploded.
Preparation[]
Inorganic azides[]
Sodium azide is made industrially by the reaction of nitrous oxide, N2O with sodium amide in liquid ammonia as solvent:[12]
- N2O + 2 NaNH2 → NaN3 + NaOH + NH3
Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia.[13] Some azides are produced by treating the carbonate salts with hydrazoic acid.
Organic azides[]
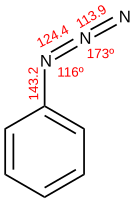
As a pseudohalide, azide generally displaces many leaving group (e.g., Br-, I-, OTs-) to give the azido compound. Aryl azides may be prepared by displacement of the appropriate diazonium salt with sodium azide or trimethylsilyl azide; nucleophilic aromatic substitution is also possible, even with chlorides. Anilines and aromatic hydrazines undergo diazotization, as do alkyl amines and hydrazines.[1]
Appropriately functionalized aliphatic compounds undergo nucleophilic substitution with sodium azide. Aliphatic alcohols give azides via a variant of the Mitsunobu reaction, with the use of hydrazoic acid.[1] Hydrazines may also form azides by reaction with sodium nitrite:[16]
- PhNHNH2 + NaNO2 → PhN3
Alkyl or aryl acyl chlorides react with sodium azide in aqueous solution to give acyl azides,[17][18] which give isocyanates in the Curtius rearrangement.
The azo-transfer compounds, trifluoromethanesulfonyl azide and imidazole-1-sulfonyl azide, are prepared from sodium azide as well. They react with amines to give the corresponding azides:
- RNH2 → RN3
Some common methods for the synthesis of alkyl azides are outlined in the following scheme.[7][19][20][21][22][23][24][25] Undoubtedly, simple nucleophilic substitution of a suitable leaving group with an azide anion remains the method of choice. The leaving group can be halide,[26] sulfonate,[27][28] and others. The azide source is most often sodium azide (NaN3), although lithium azide (LiN3), trimethylsilyl azide (TMSN3), and tributyltin azide (Bu3SnN3) have all been used.[7] Microwave[29] and enantioselective[30] modifications of the reaction are also known. Alcohols can be converted into azides in one step using 2-azido-1,3-dimethylimidazolinium hexafluorophosphate (ADMP)[31] or under Mitsunobu conditions[32] with diphenylphosphoryl azide (DPPA). Hydroxy- and amino-azides are accessible by the epoxide and aziridine ring cleavage, respectively.[33][34] Diazo transfer onto amines using trifluoromethanesulfonyl azide TfN3 and Tosyl azide (TsN3) has been reported.[35] In recent years, direct of alkenes has become increasingly popular.[36]

Dutt–Wormall reaction[]
A classic method for the synthesis of azides is the Dutt–Wormall reaction[37] in which a diazonium salt reacts with a sulfonamide first to a diazoaminosulfinate and then on hydrolysis the azide and a sulfinic acid.[38]
Reactions[]
Inorganic azides[]
Azide salts can decompose with release of nitrogen gas. The decomposition temperatures of the alkali metal azides are: NaN3 (275 °C), KN3 (355 °C), RbN3 (395 °C), and CsN3 (390 °C). This method is used to produce ultrapure alkali metals.[39]
Protonation of azide salts gives toxic hydrazoic acid in the presence of strong acids:
- H+ + N−
3 → HN3
Azide salts may react with heavy metals or heavy metal compounds to give the corresponding azides, which are more shock sensitive than sodium azide alone. They decompose with sodium nitrite when acidified. This is a method of destroying residual azides, prior to disposal.[40]
- 2 NaN3 + 2 HNO2 → 3 N2 + 2 NO + 2 NaOH
Many inorganic covalent azides (e.g., chlorine, bromine, and iodine azides) have been described.[41]
The azide anion behaves as a nucleophile; it undergoes nucleophilic substitution for both aliphatic and aromatic systems. It reacts with epoxides, causing a ring-opening; it undergoes Michael-like conjugate addition to 1,4-unsaturated carbonyl compounds.[1]
Azides can be used as precursors of the metal nitrido complexes by being induced to release N2, generating a metal complex in unusual oxidation states (see high-valent iron).
Organic azides[]
Organic azides engage in useful organic reactions. The terminal nitrogen is mildly nucleophilic. Azides easily extrude diatomic nitrogen, a tendency that is exploited in many reactions such as the Staudinger ligation or the Curtius rearrangement.[42]
Azides may be reduced to amines by hydrogenolysis[43] or with a phosphine (e.g., triphenylphosphine) in the Staudinger reaction. This reaction allows azides to serve as protected -NH2 synthons, as illustrated by the synthesis of 1,1,1-tris(aminomethyl)ethane:
- 3 H2 + CH3C(CH2N3)3 → CH3C(CH2NH2)3 + 3 N2
In the azide alkyne Huisgen cycloaddition, organic azides react as 1,3-dipoles, reacting with alkynes to give substituted 1,2,3-triazoles.
Another azide regular is tosyl azide here in reaction with norbornadiene in a nitrogen insertion reaction:[44]
Applications[]
About 250 tons of azide-containing compounds are produced annually, the main product being sodium azide.[45]
Detonators and propellants[]
Sodium azide is the propellant in automobile airbags. It decomposes on heating to give nitrogen gas, which is used to quickly expand the air bag:[45]
- 2 NaN3 → 2 Na + 3 N2
Heavy metal salts, such as lead azide, Pb(N3)2, are shock-sensitive detonators which decompose to the corresponding metal and nitrogen, for example:[46]
- Pb(N3)2 → Pb + 3 N2
Silver and barium salts are used similarly. Some organic azides are potential rocket propellants, an example being 2-dimethylaminoethylazide (DMAZ).
Other[]
Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.
Some azides are valuable as bioorthogonal chemical reporters, molecules that can be "clicked" to see the metabolic path it has taken inside a living system.
The antiviral drug zidovudine (AZT) contains an azido group.
Sodium azide is used as a biocide to prevent perturbations and artefacts from uncontrolled microbial growth in laboratory experiments (aqueous solutions, suspensions, slurries...).
Safety[]
- Azides are explosophores and toxins.
- Sodium azide is toxic (as sodium cyanide) (with an oral LD50 of 27 mg/kg in rats) and can be absorbed through the skin. It decomposes explosively upon heating to above 275 °C and reacts vigorously with CS2, bromine, nitric acid, dimethyl sulfate, and a series of heavy metals, including copper and lead. In reaction with Brønsted acids the highly toxic explosive hydrogen azide is released.
- Heavy metal azides, such as lead azide are primary high explosives detonable when heated or shaken. Heavy-metal azides are formed when solutions of sodium azide or HN3 vapors come into contact with heavy metals or their salts. Heavy-metal azides can accumulate under certain circumstances, for example, in metal pipelines and on the metal components of diverse equipment (rotary evaporators, freezedrying equipment, cooling traps, water baths, waste pipes), and thus lead to violent explosions.
- Some organic and other covalent azides are classified as highly explosive and toxic: inorganic azides as neurotoxins; azide ions, like cyanide ions, behave as cytochrome c oxidase inhibitors.
- It has been reported that sodium azide and polymer-bound azide reagents react with di- and trihalomethanes to form di- and triazidomethane respectively, which are both unstable without being handled in solutions. Various explosions have been reported during the concentration of reaction mixtures in rotary evaporators. The hazards of diazidomethane (and triazidomethane) have been well documented.[47][48]
- Solid halogen azides are very explosive and should not be prepared in the absence of solvent.[49]
See also[]
References[]
![]() This article incorporates text by Oleksandr Zhurakovskyi available under the CC BY 2.5 license.
This article incorporates text by Oleksandr Zhurakovskyi available under the CC BY 2.5 license.
- ^ Jump up to: a b c d S. Bräse; C. Gil; K. Knepper; V. Zimmermann (2005). "Organic Azides: An Exploding Diversity of a Unique Class of Compounds". Angewandte Chemie International Edition. 44 (33): 5188–5240. doi:10.1002/anie.200400657. PMID 16100733.
- ^ Griess, John Peter; Hofmann, August Wilhelm Von (1864-01-01). "XX. On a new class of compounds in which nitrogen is substituted for hydrogen". Proceedings of the Royal Society of London. 13: 375–384. doi:10.1098/rspl.1863.0082. S2CID 94746575.
- ^ Griess, Peter (1866). "Ueber eine neue Klasse organischer Verbindungen, in denen Wasserstoff durch Stickstoff vertreten ist". Annalen der Chemie und Pharmacie (in German). 137 (1): 39–91. doi:10.1002/jlac.18661370105.
- ^ Jay, R.; Curtius, Th. (January 1894). "Zur Reduction des Diazoessigesters". Berichte der Deutschen Chemischen Gesellschaft (in German). 27 (1): 775–778. doi:10.1002/cber.189402701151.
- ^ Huisgen, Rolf (October 1963). "1,3-Dipolar Cycloadditions. Past and Future". Angewandte Chemie International Edition in English. 2 (10): 565–598. doi:10.1002/anie.196305651. ISSN 0570-0833.
- ^ Huisgen, R. (November 1963). "Kinetics and Mechanism of 1,3-Dipolr Cycloadditions". Angewandte Chemie International Edition in English. 2 (11): 633–645. doi:10.1002/anie.196306331. ISSN 0570-0833.
- ^ Jump up to: a b c Organic azides: syntheses and applications. Stefan Bräse, Klaus Banert. Chichester, West Sussex, U.K.: John Wiley. 2010. ISBN 978-0-470-68252-4. OCLC 587390490.CS1 maint: others (link)
- ^ Demko, Zachary P.; Sharpless, K. Barry (November 2001). "Preparation of 5-Substituted 1 H -Tetrazoles from Nitriles in Water †". The Journal of Organic Chemistry. 66 (24): 7945–7950. doi:10.1021/jo010635w. ISSN 0022-3263. PMID 11722189.
- ^ Kolb, Hartmuth C.; Finn, M. G.; Sharpless, K. Barry (2001). "Click Chemistry: Diverse Chemical Function from a Few Good Reactions". Angewandte Chemie International Edition. 40 (11): 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. ISSN 1521-3773. PMID 11433435.
- ^ Binder, Wolfgang; Kluger, Christian (2006-09-01). "Azide/Alkyne-"Click" Reactions: Applications in Material Science and Organic Synthesis". Current Organic Chemistry. 10 (14): 1791–1815. doi:10.2174/138527206778249838.
- ^ Rostovtsev, Vsevolod V.; Green, Luke G.; Fokin, Valery V.; Sharpless, K. Barry (2002). "A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective "Ligation" of Azides and Terminal Alkynes". Angewandte Chemie International Edition. 41 (14): 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. ISSN 1521-3773. PMID 12203546.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 433. ISBN 978-0-08-037941-8.
- ^ Müller, Thomas G.; Karau, Friedrich; Schnick, Wolfgang; Kraus, Florian (2014). "A New Route to Metal Azides". Angewandte Chemie. 53 (50): 13695–13697. doi:10.1002/anie.201404561. PMID 24924913.
- ^ I. Bernal, J. Cetrullo, W. G. Jackson (1993). "The phenomenon of conglomerate crystallization in coordination compounds. XXIII: The crystallization behavior of [cis-Co(en)2(N3)(SO3)]·2H2O (I) and of [cis-Co(en)2(NO2)(SO3)]·H2O (II)". Struct.Chem. 4: 235. doi:10.1007/BF00673698. S2CID 94847897.CS1 maint: uses authors parameter (link)
- ^ . doi:10.1021/ol901122h. Cite journal requires
|journal=(help); Missing or empty|title=(help) - ^ R. O. Lindsay and C. F. H. Allen (1942). "Phenyl azide". Organic Syntheses. 22: 96. doi:10.15227/orgsyn.022.0096.
- ^ C. F. H. Allen; Alan Bell. "Undecyl isocyanate". Organic Syntheses.; Collective Volume, 3, p. 846
- ^ Jon Munch-Petersen (1963). "m-Nitrobenzazide". Organic Syntheses.; Collective Volume, 4, p. 715
- ^ Patai, Saul, ed. (1971-01-01). The Azido Group (1971). Chichester, UK: John Wiley & Sons, Ltd. p. 626. doi:10.1002/9780470771266. ISBN 978-0-470-77126-6.
- ^ Scriven, Eric F. Azides and Nitrenes: Reactivity and Utility. Academic Press. p. 542. ISBN 9780124143074.
- ^ Padwa, Albert; Pearson, William H., eds. (2002-04-05). Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products: Padwa/Dipolar Cycloaddition E-Bk. Chemistry of Heterocyclic Compounds: A Series Of Monographs. New York, USA: John Wiley & Sons, Inc. doi:10.1002/0471221902. ISBN 978-0-471-38726-8.
- ^ Wolff, H. Org. React. 1946, 3, 337–349.
- ^ Boyer, J. H.; Canter, F. C. (1954-02-01). "Alkyl and Aryl Azides". Chemical Reviews. 54 (1): 1–57. doi:10.1021/cr60167a001. ISSN 0009-2665.
- ^ Scriven, Eric F. V.; Turnbull, Kenneth (March 1988). "Azides: their preparation and synthetic uses". Chemical Reviews. 88 (2): 297–368. doi:10.1021/cr00084a001. ISSN 0009-2665.
- ^ Bräse, Stefan; Gil, Carmen; Knepper, Kerstin; Zimmermann, Viktor (2005-08-19). "Organic Azides: An Exploding Diversity of a Unique Class of Compounds". Angewandte Chemie International Edition. 44 (33): 5188–5240. doi:10.1002/anie.200400657. ISSN 1433-7851. PMID 16100733.
- ^ Righi, Giuliana; D'Achille, Claudia; Pescatore, Giovanna; Bonini, Carlo (September 2003). "New stereoselective synthesis of the peptidic aminopeptidase inhibitors bestatin, phebestin and probestin". Tetrahedron Letters. 44 (37): 6999–7002. doi:10.1016/S0040-4039(03)01799-4.
- ^ Baran, Phil S.; Zografos, Alexandros L.; O'Malley, Daniel P. (March 2004). "Short Total Synthesis of (±)-Sceptrin". Journal of the American Chemical Society. 126 (12): 3726–3727. doi:10.1021/ja049648s. ISSN 0002-7863. PMID 15038721.
- ^ Shaffer, Karl J.; Taylor, Carol M. (2006-08-01). "β-Glycosides of Hydroxyproline via an Umpolung Approach". Organic Letters. 8 (18): 3959–3962. doi:10.1021/ol061424m. ISSN 1523-7060. PMID 16928048.
- ^ Singh, Pradeep N.D.; Muthukrishnan, Sivaramakrishnan; Murthy, Rajesh S.; Klima, Rodney F.; Mandel, Sarah M.; Hawk, Michael; Yarbrough, Nina; Gudmundsdóttir, Anna D. (December 2003). "A simple and fast procedure for efficient synthesis of β- and γ-azidoarylketones". Tetrahedron Letters. 44 (51): 9169–9171. doi:10.1016/j.tetlet.2003.10.033.
- ^ Martinez, Luis E.; Leighton, James L.; Carsten, Douglas H.; Jacobsen, Eric N. (May 1995). "Highly Enantioselective Ring Opening of Epoxides Catalyzed by (salen)Cr(III) Complexes". Journal of the American Chemical Society. 117 (21): 5897–5898. doi:10.1021/ja00126a048. ISSN 0002-7863.
- ^ Kitamura, Mitsuru; Koga, Tatsuya; Yano, Masakazu; Okauchi, Tatsuo (June 2012). "Direct Synthesis of Organic Azides from Alcohols Using 2-Azido-1,3-dimethylimidazolinium Hexafluorophosphate". Synlett. 23 (9): 1335–1338. doi:10.1055/s-0031-1290958. ISSN 0936-5214.
- ^ Lee, Sang-Hyeup; Yoon, Juyoung; Chung, Seung-Hwan; Lee, Yoon-Sik (March 2001). "Efficient asymmetric synthesis of 2,3-diamino-3-phenylpropanoic acid derivatives". Tetrahedron. 57 (11): 2139–2145. doi:10.1016/S0040-4020(01)00090-4.
- ^ Sabitha, Gowravaram; Babu, R. Satheesh; Rajkumar, M.; Yadav, J. S. (February 2002). "Cerium(III) Chloride Promoted Highly Regioselective Ring Opening of Epoxides and Aziridines Using NaN 3 in Acetonitrile: A Facile Synthesis of 1,2-Azidoalcohols and 1,2-Azidoamines †". Organic Letters. 4 (3): 343–345. doi:10.1021/ol016979q. ISSN 1523-7060. PMID 11820875.
- ^ Saito, Seiki; Bunya, Norio; Inaba, Masami; Moriwake, Toshio; Torii, Sigeru (January 1985). "A facile cleavage of oxirane with hydrazoic acid in dmf A new route to chiral β-hydroxy-α-amino acids". Tetrahedron Letters. 26 (43): 5309–5312. doi:10.1016/S0040-4039(00)95024-X.
- ^ Titz, Alexander; Radic, Zorana; Schwardt, Oliver; Ernst, Beat (April 2006). "A safe and convenient method for the preparation of triflyl azide, and its use in diazo transfer reactions to primary amines". Tetrahedron Letters. 47 (14): 2383–2385. doi:10.1016/j.tetlet.2006.01.157.
- ^ Waser, Jérôme; Gaspar, Boris; Nambu, Hisanori; Carreira, Erick M. (2006-09-01). "Hydrazines and Azides via the Metal-Catalyzed Hydrohydrazination and Hydroazidation of Olefins". Journal of the American Chemical Society. 128 (35): 11693–11712. doi:10.1021/ja062355+. ISSN 0002-7863. PMID 16939295.
- ^ Pavitra Kumar Dutt; Hugh Robinson Whitehead & Arthur Wormall (1921). "CCXLI.—The action of diazo-salts on aromatic sulphonamides. Part I". J. Chem. Soc., Trans. 119: 2088–2094. doi:10.1039/CT9211902088.
- ^ Name Reactions: A Collection of Detailed Reaction Mechanisms by Jie Jack Li Published 2003 Springer ISBN 3-540-40203-9
- ^ E. Dönges "Alkali Metals" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 475.
- ^ Committee on Prudent Practices for Handling, Storage, and Disposal of Chemicals in Laboratories, Board on Chemical Sciences and Technology, Commission on Physical Sciences, Mathematics, and Applications, National Research Council (1995). Prudent practices in the laboratory: handling and disposal of chemicals. Washington, D.C.: National Academy Press. ISBN 0-309-05229-7.CS1 maint: multiple names: authors list (link)
- ^ I. C. Tornieporth-Oetting & T. M. Klapötke (1995). "Covalent Inorganic Azides". Angewandte Chemie International Edition in English. 34 (5): 511–520. doi:10.1002/anie.199505111.
- ^ Saul Patai, ed. (1971). The Azido Group. PATAI'S Chemistry of Functional Groups. doi:10.1002/9780470771266.
- ^ "Amine synthesis by azide reduction".
- ^ Damon D. Reed & Stephen C. Bergmeier (2007). "A Facile Synthesis of a Polyhydroxylated 2-Azabicyclo[3.2.1]octane". J. Org. Chem. 72 (3): 1024–6. doi:10.1021/jo0619231. PMID 17253828.
- ^ Jump up to: a b Horst H. Jobelius, Hans-Dieter Scharff "Hydrazoic Acid and Azides" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_193
- ^ Shriver and Atkins. Inorganic Chemistry (Fifth Edition). W. H. Freeman and Company, New York, pp 382.
- ^ M. S. Alfred Hassner (1986). "Synthesis of Alkyl Azides with a Polymeric Reagent". Angewandte Chemie International Edition in English. 25 (5): 478–479. doi:10.1002/anie.198604781.
- ^ A. Hassner; M. Stern; H. E. Gottlieb; F. Frolow (1990). "Synthetic methods. 33. Utility of a polymeric azide reagent in the formation of di- and triazidomethane. Their NMR spectra and the x-ray structure of derived triazoles". J. Org. Chem. 55 (8): 2304–2306. doi:10.1021/jo00295a014.
- ^ L. Marinescu; J. Thinggaard; I. B. Thomsen; M. Bols (2003). "Radical Azidonation of Aldehydes". J. Org. Chem. 68 (24): 9453–9455. doi:10.1021/jo035163v. PMID 14629171.
External links[]
| Look up azide or azido- in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Azides. |
- Functional groups
- Azides
- Leaving groups