Potassium azide
|
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Potassium azide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.039.997 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| KN 3 | |||
| Molar mass | 81.1184 g/mol | ||
| Appearance | Colorless crystals[1] | ||
| Density | 2.038 g/cm3 [1] | ||
| Melting point | 350 °C (662 °F; 623 K) (in vacuum)[1] | ||
| Boiling point | decomposes | ||
| 41.4 g/100 mL (0 °C) 50.8 g/100 mL (20 °C) 105.7 g/100 mL (100 °C) | |||
| Solubility | soluble in ethanol insoluble in ether | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
-1.7 kJ/mol | ||
| Hazards | |||
| Main hazards | Very Toxic, explosive if strongly heated | ||
| NFPA 704 (fire diamond) | 
4
3
3 | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
27 mg/kg (oral, rat)[2] | ||
| Related compounds | |||
Other cations
|
Sodium azide, copper(II) azide, lead(II) azide, silver azide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Potassium azide is the inorganic compound having the formula KN
3. It is a white, water-soluble salt. It is used as a reagent in the laboratory.
It has been found to act as a nitrification inhibitor in soil.[3]
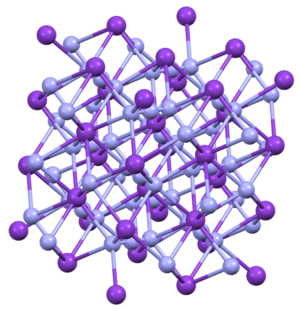
Structure[]
KN3, RbN3, CsN3, and TlN3 adopt the same structures. They crystallize in a tetragonal habit.[4] The azide is bound to eight cations in an eclipsed orientation. The cations are bound to eight terminal N centers.[5]

Synthesis and reactions[]
KN3 is prepared by treating potassium carbonate with hydrazoic acid, which is generated in situ.[6] In contrast, the analogous sodium azide is prepared (industrially) by the "Wislicenus process," which proceeds via the reaction sodium amide with nitrous oxide.[7]
Upon heating or upon irradiation with ultraviolet light, it decomposes into potassium metal and nitrogen gas.[8] The decomposition temperatures of the alkali metal azides are: NaN3 (275 °C), KN3 (355 °C), RbN3 (395 °C), CsN3 (390 °C).[9]
Health hazards[]
Like sodium azide, potassium azide is very toxic. The TLV of the related sodium azide is 0.07 ppm. The toxicity of azides arise from their ability to inhibit cytochrome c oxidase.[7]
References[]
- ^ a b c Dale L. Perry; Sidney L. Phillips (1995). Handbook of inorganic compounds. CRC Press. p. 301. ISBN 0-8493-8671-3.
- ^ "Substance Name: Potassium azide". chem.sis.nlm.nih.gov. Archived from the original on 2014-08-12. Retrieved 2014-08-11.
- ^ T. D. Hughes; L. F. Welch (1970). "Potassium Azide as a Nitrification Inhibitor". Agronomy Journal. American Society of Agronomy. 62 (5): 595–599. doi:10.2134/agronj1970.00021962006200050013x.
- ^ Khilji, M. Y.; Sherman, W. F.; Wilkinson, G. R. (1982). "Variable temperature and pressure Raman spectra of potassium azide KN
3". Journal of Raman Spectroscopy. 12 (3): 300–303. Bibcode:1982JRSp...12..300K. doi:10.1002/jrs.1250120319. - ^ Ulrich Müller "Verfeinerung der Kristallstrukturen von KN3, RbN3, CsN3 und TIN3" Zeitschrift für anorganische und allgemeine Chemie 1972, Volume 392, 159–166. doi:10.1002/zaac.19723920207
- ^ P. W. Schenk "Alkali Azides from Carbonates" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 475.
- ^ a b Horst H. Jobelius, Hans-Dieter Scharff "Hydrazoic Acid and Azides" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_193
- ^ Tompkins, F. C.; Young, D. A. (1982). "The Photochemical and Thermal Formation of Colour Centres in Potassium Azide Crystals". Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences. 236 (1204): 10–23.
- ^ E. Dönges "Alkali Metals" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 475.
- Azides
- Potassium compounds