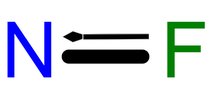
Nitrogen monofluoride
| |
| |
| Names | |
|---|---|
| Other names
Fluoroimidogen
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| FN | |
| Molar mass | 33.005 g·mol−1 |
| Related compounds | |
Related isoelectronic
|
Dioxygen, |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitrogen monofluoride (fluoroimidogen) is a metastable species that has been observed in laser studies. It is isoelectronic with O2. Like boron monofluoride, it has unusual higher than single-bonded fluorine.[1][2] It is unstable with respect to its formal dimer, dinitrogen difluoride, as well as to its elements, nitrogen and fluorine. Nitrogen monofluoride can be formed by the decomposition of fluorine azide into N2F2 and N2. It is also produced when various radical species (H, O, N, CH3) react with nitrogen difluoride (NF2) to abstract one fluorine atom.[3] Many of the reactions give the product in an excited state that has a characteristic chemiluminescence, and have thus been investigated for development as a chemical laser.[4][5] The reactions are highly efficient and the product has a long lifetime. The reaction with molecular hydrogen (H2) involves a chain propagation via regeneration of the atomic hydrogen radical that can perpetuate for many cycles. An alternative azide process involves the reaction of atomic fluorine with hydrazoic acid to give an azide radical, which then reacts with another atomic fluorine to produce NF with N2 as a byproduct. This route avoids the necessity of using atomic hydrogen, a chemical that could otherwise cause the decomposition of NF.[5]
References[]
- ^ http://metastablestates.com/Publications/JPC_93_1078_1989.pdf
- ^ Harbison, G. S. (2002). "The Electric Dipole Polarity of the Ground and Low-lying Metastable Excited States of NF". Journal of the American Chemical Society. 124 (3): 366–367. doi:10.1021/ja0159261. PMID 11792193.
- ^ Gmelin-lnstitut für Anorganische Chemie der Max-Planck-Gesellschaft zur Förderung der Wissenschaften (2013). Gmelin Handbook of Inorganic Chemistry: F Fluorine: Compounds with Oxygen and Nitrogen. Springer Science & Business Media. pp. 263–271. ISBN 9783662063392.
- ^ Kenner, Rex D.; Ogryzlo, Elmer A. (1985). "Chemiluminescence in Gas Phase Reactions; 4. NF(a1Δ) (870, 875 nm) and (b1Σ+) (525–530 nm)". In Burr, John G. (ed.). Chemi- and Bioluminescence. Chemical and Biochemical Analysis. 16. Dekker. pp. 84–87. ISBN 0-8247-7277-6.
- ^ a b Avizonis, Petras V. (2012). "Chemically Pumped Electronic Transition Lasers". In Onorato, Michele (ed.). Gas Flow and Chemical Lasers. Plenum Press. pp. 1–19. ISBN 978-1-4615-7067-7.
- Nitrogen fluorides
- Nitrogen compounds