Ruthenium pentafluoride
| |

| |
| Names | |
|---|---|
| IUPAC name
ruthenium(V) fluoride
| |
| Other names
Ruthenium(V) fluoride, Ruthenium(5+) pentafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.035.015 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| F5Ru | |
| Molar mass | 196.06 |
| Appearance | green solid |
| Density | 3.82 g/cm3 |
| Melting point | 86.5 °C (187.7 °F; 359.6 K) |
| Boiling point | 227 °C (441 °F; 500 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
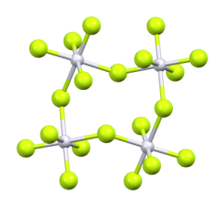
Ruthenium pentafluoride is the inorganic compound with the empirical formula RuF5. This green volatile solid has rarely been studied but is of interest as a binary fluoride of ruthenium, i.e. a compound containing only Ru and F. It is sensitive toward hydrolysis. Its structure consists of Ru4F20 tetramers, as seen in the isostructural platinum pentafluoride. Within the tetramers, each Ru adopts octahedral molecular geometry, with two bridging fluoride ligands.[1]
References[]
- ^ J. H. Holloway, R. D. Peacock, R. W. H. Small "The crystal structure of ruthenium pentafluoride" J. Chem. Soc., 1964, 644-648. doi:10.1039/JR9640000644
Categories:
- Ruthenium compounds
- Fluorides
- Platinum group halides