Titanium tetrafluoride
| |
| Names | |
|---|---|
| IUPAC name
Titanium(IV) fluoride
| |
| Other names
Titanium tetrafluoride
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.106 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
Chemical formula
|
TiF4 |
| Molar mass | 123.861 g/mol |
| Appearance | white powder |
| Density | 2.798 g/cm3 |
| Melting point | 377 °C (711 °F; 650 K) |
| Boiling point | sublimes |
| Hazards | |
| GHS labelling:[1] | |
 
| |
Signal word
|
Danger |
| H302, H312, H314, H332 | |
Precautionary statements
|
P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P322, P330, P363, P405 |
| NFPA 704 (fire diamond) | 
3
0
0 |
| Related compounds | |
Other anions
|
Titanium(IV) bromide Titanium(IV) chloride Titanium(IV) iodide |
Related compounds
|
Titanium(III) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
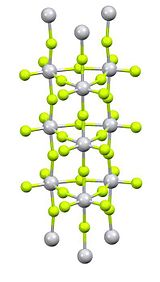
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure.[2] In common with the other tetrahalides, TiF4 is a strong Lewis acid.
Preparation, structure, reactions[]
The traditional method involves treatment of titanium tetrachloride with excess hydrogen fluoride:
- TiCl4 + 4 HF → TiF4 + 4 HCl
Purification is by sublimation, which involves reversible cracking of the polymeric structure.[3] X-ray crystallography reveals that the Ti centres are octahedral, but conjoined in an unusual columnar structure.[4]
TiF4 forms adducts with many ligands. One example is cis-TiF4(MeCN)2, which is formed by treatment with acetonitrile.[5]
References[]
- ^ "Titanium tetrafluoride". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 200.
- ^ Bialowons, H.; Mueller, M.; Mueller, B.G. (1995). "Titantetrafluorid - Eine Überraschend einfache Kolumnarstruktur". Zeitschrift für Anorganische und Allgemeine Chemie. 621: 1227–1231. doi:10.1002/zaac.19956210720.
- ^ Nikiforov, Grigory B.; Roesky, Herbert W.; Koley, Debasis (2014). "A survey of titanium fluoride complexes, their preparation, reactivity, and applications". Coordination Chemistry Reviews. 258–259: 16–57. doi:10.1016/j.ccr.2013.09.002.
Categories:
- Fluorides
- Metal halides
- Titanium(IV) compounds
- Inorganic compound stubs