Uranyl fluoride
| |
| Names | |
|---|---|
| IUPAC name
Uranium fluoride oxide
| |
| Other names
Uranium oxyfluoride
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.529 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| UO2F2 | |
| Molar mass | 308.02 g/mol |
| Melting point | Decomposes @ 300 °C |
| Boiling point | Sublimes |
| Solubility in other solvents | VS |
| Hazards | |
| GHS labelling: | |
  
| |
Signal word
|
Danger |
| H300, H330, H373, H411 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
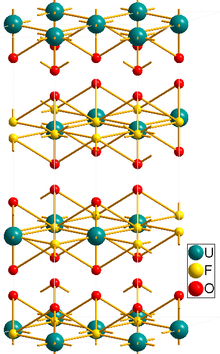
Uranyl fluoride is the inorganic compound with the formula UO2F2. As shown by x-ray crystallography, the uranyl (UO22+) centers are complemented by six fluoride ligands.[1]
It is very soluble in water as well as hygroscopic. It is forms in the hydrolysis of uranium hexafluoride (UF6):
- UF6 + 2 H2O → UO2F2 + 4 HF.
References[]
- ^ Zachariasen, W. H. (1948). "Crystal chemical studies of the 5f-series of elements. III. A study of the disorder in the crystal structure of anhydrous uranyl fluoride". Acta Crystallographica. 1 (6): 277–281. doi:10.1107/S0365110X48000764.
Categories:
- Uranyl compounds
- Fluorides
- Metal halides
- Oxohalides
- Inorganic compound stubs