Zirconium tetrafluoride

| |
| Names | |
|---|---|
| IUPAC names
Zirconium(IV) fluoride
Zirconium tetrafluoride | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.107 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
Chemical formula
|
ZrF4 |
| Molar mass | 167.21 g/mol |
| Appearance | white crystalline powder |
| Density | 4.43 g/cm3 (20 °C) |
| Melting point | 910 °C (1,670 °F; 1,180 K) |
| 1.32 g/100mL (20 °C) 1.388 g/100mL (25 °C) | |
| Structure | |
| Monoclinic, mS60 | |
Space group
|
C12/c1, No. 15 |
| Hazards | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
98 mg/kg (oral, mouse) 98 mg/kg (oral, rat)[1] |
| Related compounds | |
Other anions
|
Zirconium(IV) chloride Zirconium(IV) bromide Zirconium(IV) iodide |
Other cations
|
Titanium(IV) fluoride Hafnium(IV) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zirconium(IV) fluoride (ZrF4) is an inorganic chemical compound. It is a component of ZBLAN fluoride glass. It is insoluble in water. It is the main component of fluorozirconate glasses.
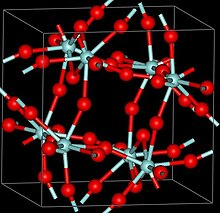
Three crystalline phases of ZrF4 have been reported, α (monoclinic), β (tetragonal, Pearson symbol tP40, space group P42/m, No 84) and γ (unknown structure). β and γ phases are unstable and irreversibly transform into the α phase at 400 °C.[2]
Zirconium fluoride is used as a zirconium source in oxygen-sensitive applications, e.g. metal production.[3] Zirconium fluoride can be purified by distillation or sublimation.[4]
Conditions/substances to avoid are: moisture, active metals, acids and oxidizing agents.
Zirconium fluoride in a mixture with other fluorides is a coolant for molten salt reactors. In the mixture with sodium fluoride it is a candidate coolant for the Advanced High-Temperature Reactor.
Together with uranium salt, zirconium fluoride can be a component of fuel-coolant in molten salt reactors. Mixture of sodium fluoride, zirconium fluoride, and uranium tetrafluoride (53-41-6 mol.%) was used as a coolant in the Aircraft Reactor Experiment. A mixture of lithium fluoride, beryllium fluoride, zirconium fluoride, and uranium-233 tetrafluoride was used in the Molten-Salt Reactor Experiment. (Uranium-233 is used in the thorium fuel cycle reactors.)
References[]
- ^ "Zirconium compounds (as Zr)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Paul L. Brown; Federico J. Mompean; Jane Perrone; Myriam Illemassène (2005). Chemical thermodynamics of zirconium. Gulf Professional Publishing. p. 144. ISBN 0-444-51803-7.
- ^ "Zirconium fluoride". American Elements. Retrieved 2009-07-07.
- ^ "Method for preparing ultra-pure zirconium and hafnium tetrafluorides. United States Patent 4578252". Retrieved 2009-07-07.
- ORNL/TM-2006/12 Assessment of Candidate Molten Salt Coolants for the Advanced High-Temperature Reactor (AHTR), March 2006 (Accessed 2008/9/18)
- Fluorides
- Zirconium compounds
- Metal halides
- Inorganic compound stubs