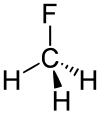
Fluoromethane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Fluoromethane
| |||
| Other names
Freon 41
Methyl fluoride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | R41 | ||
| 1730725 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.907 | ||
| EC Number |
| ||
| 391 | |||
| KEGG | |||
| MeSH | Fluoromethane | ||
PubChem CID
|
|||
| UNII | |||
| UN number | UN 2454 | ||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
Chemical formula
|
CH3F | ||
| Molar mass | 34.03 g/mol | ||
| Appearance | Colourless gas | ||
| Odor | pleasant, ether-like odour at high concentrations | ||
| Density | 1.4397 g/L 0.557 g/cm3 (liquid) at saturation pressure at 25 °C | ||
| Melting point | −137.8 °C (−216.0 °F; 135.3 K) [1] | ||
| Boiling point | −78.4 °C (−109.1 °F; 194.8 K) [1] | ||
| 1.66 L/kg (2.295 g/L) | |||
| Vapor pressure | 3.3 MPa | ||
| Hazards | |||
EU classification (DSD) (outdated)
|
|||
| R-phrases (outdated) | R12 | ||
| S-phrases (outdated) | S9, S16, S23, S24/25, S26, S28, S33, S36/37/39, S60 | ||
| NFPA 704 (fire diamond) | 
1
4
0 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) with a fluorine atom substituted for one of the hydrogen atoms. It is used in semiconductor manufacturing processes as an etching gas in plasma etch reactors.[2]
Composition[]
The compound is the lowest mass member of the hydrofluorocarbon (HFC) family, compounds which contain only hydrogen, fluorine, and carbon. These compounds are related to the chlorofluorocarbons (CFC), but since they do not contain chlorine, are not destructive to the ozone layer.[3] Fluorocarbons are, however, potent greenhouse gasses, and the Kigali Amendment to the Montreal Protocol is an attempt to phase them out due to their contribution to global warming.[4]
The C−F bond energy is 552 kJ/mol and its length is 0.139 nm (typically 0.14 nm). Its molecular geometry is tetrahedral.
Its specific heat capacity (Cp) is 38.171 J·mol−1·K−1 at 25 °C. The critical point of fluoromethane is at 44.9 °C (318.1 K) and 6.280 MPa.
See also[]
- Organofluorine chemistry
- Halomethanes
References[]
- ^ Jump up to: a b Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- ^ Fluoromethane CH3F
- ^ "Explainer: hydrofluorocarbons saved the ozone layer, so why are we banning them?".
External links[]
- MSDS at Chemblink.com[permanent dead link]
- Data at Airliquide Encyclopedia[permanent dead link]
- Thermochemical data at chemnet.ru
- CAS DataBase List METHYL FLUORIDE at ChemicalBook
- Fluoroalkanes
- Halomethanes
- Refrigerants
- Gases