Thallium(I) fluoride
| |
| Names | |
|---|---|
| Preferred IUPAC name
Thallium(I) fluoride[citation needed] | |
| Other names
Thallium monofluoride[citation needed]
Thallous fluoride[citation needed] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.231 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| Properties | |
| TlF | |
| Molar mass | 223.3817 g/mol |
| Appearance | White crystals |
| Density | 8.36 g cm−3 |
| Melting point | 327 °C (621 °F; 600 K) |
| Boiling point | 655 °C (1,211 °F; 928 K) (decomposes) |
| 78.6 g/100 mL (at 15 °C)[1] | |
| Solubility | slightly soluble in ethanol |
Magnetic susceptibility (χ)
|
−44.4·10−6 cm3/mol |
| Structure | |
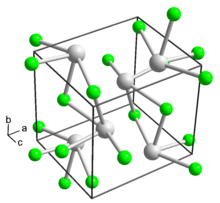
| Orthorhombic, oP8 | |
Space group
|
Fmmm, No. 28 |
| Hazards | |
EU classification (DSD) (outdated)
|
|
| R-phrases (outdated) | R26/28, R33, R51/53 |
| S-phrases (outdated) | S13, S28, S45, S61[2] |
| Related compounds | |
Other anions
|
Thallium(I) chloride |
Other cations
|
Gallium(III) fluoride Indium(III) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thallium(I) fluoride (or thallous fluoride or thallium monofluoride) is the chemical compound composed of thallium and fluorine with the formula TlF. It consists of hard white orthorhombic crystals which are slightly deliquescent in humid air but revert to the anhydrous form in dry air.[1] It has a distorted sodium chloride (rock salt) crystal structure,[3][4] due to the 6s2 inert pair on Tl+.[5]
Thallium(I) fluoride is unusual among the thallium(I) halides in that it is very soluble in water, while the others are not.[6]
Reactions[]
Thallium(I) fluoride can be prepared by the reaction of thallium(I) carbonate with hydrofluoric acid.[3]
At 928 K, TlF decomposes to its elements:
References[]
- ^ Jump up to: a b Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 407, ISBN 0-8493-8671-3, retrieved 2008-06-17
- ^ "399833 Thallium(I) fluoride 99%". Sigma-Aldrich. Retrieved 2008-06-17.
- ^ Jump up to: a b Wiberg, Nils; Wiberg, Egon; Holleman, A. F. (2001), Inorganic Chemistry, Academic Press, p. 1037, ISBN 0-12-352651-5, retrieved 2008-06-17
- ^ Meyer, Gerd; Naumann, Dieter; Wesemann, Lars (2006), Inorganic Chemistry in Focus III, Wiley-VCH, p. 21, ISBN 3-527-31510-1, retrieved 2008-06-17
- ^ Berastegui, P.; Hull, S. (2000). "The Crystal Structures of Thallium(I) Fluoride". Journal of Solid State Chemistry. 150 (2): 266. doi:10.1006/jssc.1999.8587.
- ^ Arora, M. G. (2003), P-block Elements, Anmol Publications, p. 35, ISBN 81-7488-563-3, retrieved 2008-06-17
- ^ https://www.americanelements.com/thallium-i-fluoride-7789-27-7
Categories:
- Thallium(I) compounds
- Fluorides
- Metal halides
