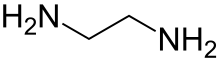
Ethylenediamine
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ethane-1,2-diamine[2] | |||
| Other names
Edamine,[1] 1,2-Diaminoethane, 'en' when a ligand
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| Abbreviations | en | ||
| 605263 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.154 | ||
| EC Number |
| ||
| 1098 | |||
| KEGG | |||
| MeSH | ethylenediamine | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1604 | ||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| C2H8N2 | |||
| Molar mass | 60.100 g·mol−1 | ||
| Appearance | Colorless liquid[3] | ||
| Odor | Ammoniacal[3] | ||
| Density | 0.90 g/cm3[3] | ||
| Melting point | 8 °C (46 °F; 281 K)[3] | ||
| Boiling point | 116 °C (241 °F; 389 K)[3] | ||
| miscible | |||
| log P | −2.057 | ||
| Vapor pressure | 1.3 kPa (at 20 °C) | ||
Henry's law
constant (kH) |
5.8 mol Pa−1 kg−1 | ||
| |||
Refractive index (nD)
|
1.4565 | ||
| Thermochemistry | |||
Heat capacity (C)
|
172.59 J K−1 mol−1 | ||
Std molar
entropy (S |
202.42 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−63.55–−62.47 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−1.8678–−1.8668 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |    
| ||
| GHS Signal word | Danger | ||
GHS hazard statements
|
H226, H311, H302, H332, H314, H334, H317, H412 | ||
| P405, P102, P260, P280, P305+351+338, P101, P308+313, P273, P501 | |||
| NFPA 704 (fire diamond) | 
4
3
2 | ||
| Flash point | 34 °C (93 °F; 307 K)[3] | ||
| 385 °C (725 °F; 658 K)[3] | |||
| Explosive limits | 2.7–16% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
500 mg/kg (oral, rat) 470 mg/kg (oral, guinea pig) 1160 mg/kg (oral, rat)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 10 ppm (25 mg/m3)[4] | ||
REL (Recommended)
|
TWA 10 ppm (25 mg/m3)[4] | ||
IDLH (Immediate danger)
|
1000 ppm[4] | ||
| Related compounds | |||
Related alkanamines
|
1,2-Diaminopropane, 1,3-Diaminopropane | ||
Related compounds
|
Ethylamine, Ethylenedinitramine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998.[6] Ethylenediamine readily reacts with moisture in humid air to produce a corrosive, toxic and irritating mist, to which even short exposures can cause serious damage to health (see safety). Ethylenediamine is the first member of the so-called polyethylene amines.
Synthesis[]
Ethylenediamine is produced industrially by treating 1,2-dichloroethane with ammonia under pressure at 180 °C in an aqueous medium:[6][7]
In this reaction hydrogen chloride is generated, which forms a salt with the amine. The amine is liberated by addition of sodium hydroxide and can then be recovered by rectification. Diethylenetriamine (DETA) and triethylenetetramine (TETA) are formed as by-products.
Another industrial route to ethylenediamine involves the reaction of ethanolamine and ammonia:[8]
This process involves passing the gaseous reactants over a bed of nickel heterogeneous catalysts.
It can be produced in the lab by the reaction of ethylene glycol and urea.
Ethylenediamine can be purified by treatment with sodium hydroxide to remove water followed by distillation.[9]
Applications[]
Ethylenediamine is used in large quantities for production of many industrial chemicals. It forms derivatives with carboxylic acids (including fatty acids), nitriles, alcohols (at elevated temperatures), alkylating agents, carbon disulfide, and aldehydes and ketones. Because of its bifunctional nature, having two amines, it readily forms heterocycles such as imidazolidines.
Precursor to chelation agents, drugs, and agrochemicals[]
A most prominent derivative of ethylenediamine is the chelating agent EDTA, which is derived from ethylenediamine via a Strecker synthesis involving cyanide and formaldehyde. Hydroxyethylethylenediamine is another commercially significant chelating agent.[6] Numerous bio-active compounds and drugs contain the N-CH2-CH2-N linkage, including some antihistamines.[10] Salts of ethylenebisdithiocarbamate are commercially significant fungicides under the brand names Maneb, Mancozeb, Zineb, and Metiram. Some imidazoline-containing fungicides are derived from ethylenediamine.[6]
Pharmaceutical ingredient[]
Ethylenediamine is an ingredient in the common bronchodilator drug aminophylline, where it serves to solubilize the active ingredient theophylline. Ethylenediamine has also been used in dermatologic preparations, but has been removed from some because of causing contact dermatitis.[11] When used as a pharmaceutical excipient, after oral administration its bioavailability is about 0.34, due to a substantial first-pass effect. Less than 20% is eliminated by renal excretion.[12]
Ethylenediamine-derived antihistamines are the oldest of the five classes of , beginning with piperoxan aka benodain, discovered in 1933 at the Pasteur Institute in France, and also including mepyramine, tripelennamine, and antazoline. The other classes are derivatives of ethanolamine, alkylamine, piperazine, and others (primarily tricyclic and tetracyclic compounds related to phenothiazines, tricyclic antidepressants, as well as the cyproheptadine-phenindamine family)
Role in polymers[]
Ethylenediamine, because it contains two amine groups, is a widely used precursor to various polymers. Condensates derived from formaldehyde are plasticizers. It is widely used in the production of polyurethane fibers. The PAMAM class of dendrimers are derived from ethylenediamine.[6]
Tetraacetylethylenediamine[]
The bleaching activator tetraacetylethylenediamine is generated from ethylenediamine. The derivative N,N-ethylenebis(stearamide) (EBS) is a commercially significant mold-release agent and a surfactant in gasoline and motor oil.
Other applications[]
- as a solvent, it is miscible with polar solvents and is used to solubilize proteins such as albumins and casein. It is also used in certain electroplating baths.
- as a corrosion inhibitor in paints and coolants.
- ethylenediamine dihydroiodide (EDDI) is added to animal feeds as a source of iodide.
- chemicals for color photography developing, binders, adhesives, fabric softeners, curing agents for epoxies, and dyes.
- as a compound to sensitize nitromethane into an explosive. This mixture was used at Picatinny Arsenal during WWII, giving the nitromethane and ethylenediamine mixture the nickname PLX, or Picatinny Liquid Explosive.
Coordination chemistry[]
Ethylenediamine is a well-known bidentate chelating ligand for coordination compounds, with the two nitrogen atoms donating their lone pairs of electrons when ethylenediamine acts as a ligand. It is often abbreviated "en" in inorganic chemistry. The complex [Co(ethylenediamine)3]3+ is an archetypical chiral tris-chelate complex. The salen ligands, some of which are used in catalysis, are derived from the condensation of salicylaldehydes and ethylenediamine.
Related ligands[]
Related derivatives of ethylenediamine include ethylenediaminetetraacetic acid (EDTA), tetramethylethylenediamine (TMEDA), and (TEEDA). Chiral analogs of ethylenediamine include 1,2-diaminopropane and trans-diaminocyclohexane.
Safety[]
Ethylenediamine, like ammonia and other low-molecular weight amines, is a skin and respiratory irritant. Unless tightly contained, liquid ethylenediamine will release toxic and irritating vapors into its surroundings, especially on heating. The vapors react with moisture in humid air to form a characteristic white mist, which is extremely irritating to skin, eyes, lungs and mucus membranes.
References[]
- ^ "32007R0129". European Union. 12 February 2007. Annex II. Retrieved 3 May 2012.
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 676. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Jump up to: a b c d e f g Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Jump up to: a b c NIOSH Pocket Guide to Chemical Hazards. "#0269". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Ethylenediamine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Jump up to: a b c d e Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke "Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, 2005 Wiley-VCH Verlag, Weinheim. doi:10.1002/14356007.a02_001
- ^ Hans-Jürgen Arpe, Industrielle Organische Chemie, 6. Auflage (2007), Seite 245, Wiley VCH
- ^ Hans-Jürgen Arpe, Industrielle Organische Chemie, 6. Auflage (2007), Seite 275, Wiley VCH
- ^ Rollinson, Carl L.; Bailar, Jr., John C. (1946). "Tris(ethylenediamine)chromium(III) Salts". Inorganic Syntheses. Inorganic Syntheses. 2. pp. 196–200. doi:10.1002/9780470132333.ch60. ISBN 9780470132333.
- ^ Kotti, S. R. S. S.; Timmons, C. & Li, G. (2006). "Vicinal diamino functionalities as privileged structural elements in biologically active compounds and exploitation of their synthetic chemistry". Chemical Biology & Drug Design. 67 (2): 101–114. doi:10.1111/j.1747-0285.2006.00347.x. PMID 16492158.
- ^ Hogan DJ (January 1990). "Allergic contact dermatitis to ethylenediamine. A continuing problem". Dermatol Clin. 8 (1): 133–6. doi:10.1016/S0733-8635(18)30536-9. PMID 2137392.
- ^ Zuidema J. (1985-08-23). "Ethylenediamine, profile of a sensitizing excipient". Pharmacy World & Science. 7 (4): 134–40. doi:10.1007/BF02097249. PMID 3900925.
External links[]
![]() Media related to Ethylenediamine at Wikimedia Commons
Media related to Ethylenediamine at Wikimedia Commons
- Diamines
- Amine solvents
- Chelating agents
- Fuel antioxidants
- Corrosion inhibitors
- Commodity chemicals
- Ethyleneamines
- Foul-smelling chemicals

